Clinical and Genetic Study of a XX (SRY negative) Male.
Rima Dada, M. E. Ahmad, Rashmi Talwar and Kiran Kucheria*
Genetics Division, Dept. of Anatomy,
All India Institute of Medical Sciences, New Delhi-110029, India
*Corresponding Author:
Dr. Kiran Kucheria
Professor and Head, Dept. of Anatomy
All India Institute of Medical Sciences
New Delhi-110029, INDIA
Email: kkucheria@hotmail.com
Phone: 91-11-6593489/6593216/6589089
Fax; 91-11-6862663
SUMMARY
The 46, XX male syndrome is a rare sex chromosomal disorder in man [1]. It mostly occurs due to unequal crossing over between X and Y chromosomes during meiosis [2]. A 32 year old XX male with normal male phenotype and normal adrenarchal hair pattern, bilateral atrophic testes, normal phallus presented with azoospermia and infertility. The gonadotrophins were elevated. Cytogenetic and molecular analysis using FISH with X and SRY probe showed a 46,XX (SRY negative) chromosomal constitution.
Key Words: Sex determining region on Y chromosome (SRY), Fluorescent in situ hybridization (FISH), Azoospermia, Male infertility, XX Male.
INTRODUCTION
Genetic analysis of sex reversed subjects with a phenotypic sex different from
that in the karyotype has been important in the identification and characterization
of the sex determining region on the Y chromosome (SRY). SRY is the candidate
gene for the testis determination. It resides within 35 Kilobase region of the
Y chromosome immediately proximal to the Pseudoautosomal region I (PAR I). When
the SRY gene is present, testes are formed and in its absence gonads develop
into ovaries.
46, XX males are distinct from XX true hermaphrodite and have a male habitus,
small testes, azoospermia with no evidence of uterus or ovaries [3,4]. The incidence
is about 1 in 20,000 - 25,000 male births. Different pathogenic mechanism have
been suggested which can lead to 46,XX sex reversal. These may be recombination
between X and Y chromosomes [2], mosaicism with a prevalent XX lineage and a
hidden cryptic lineage containing a Y chromosome [5], and mutation in genes
other than TDF can trigger testis determination in XX (SRY negative) males [6].
We describe here a case of 46,XX infertile azoospermic male with normal male
phenotypes showing the absence of SRY region on Fluorescent In Situ Hybridization
(FISH) analysis. We have done molecular analysis using FISH technique, which
not only detects the SRY region but also identifies the site of chromosome translocation
if any.
CASE REPORT
A 32 year old male was referred to us for cytogenetic analysis from the infertility
clinic at All India Institute of Medical Sciences (AIIMS), New Delhi. At the
time of presentation he had been married for 4 years and cohabitation since
then. He was a product of full term normal vaginal delivery from a non-consanguineous
marriage. His childhood development was normal and uneventful. There was no
history of any major illness like TB, mumps or injury to testis. There was no
family history of sex reversal.
The patients height was 5 feet 4" and had normal facial, axillary hair
and scant pubic hair.
Figure
1A: Phenotype of 46, XX Male - removed |
 |
 |
The patient's penile length was normal but both testes were soft, small and atrophic. Ultrasonogram showed the right testis as 23x17x12mm and left testis as 24x19x14mm in volume. Semen analysis was done which showed complete absence of sperms in semen confirming azoospermia [7]. The semen volume was 3ml and was fructose positive.
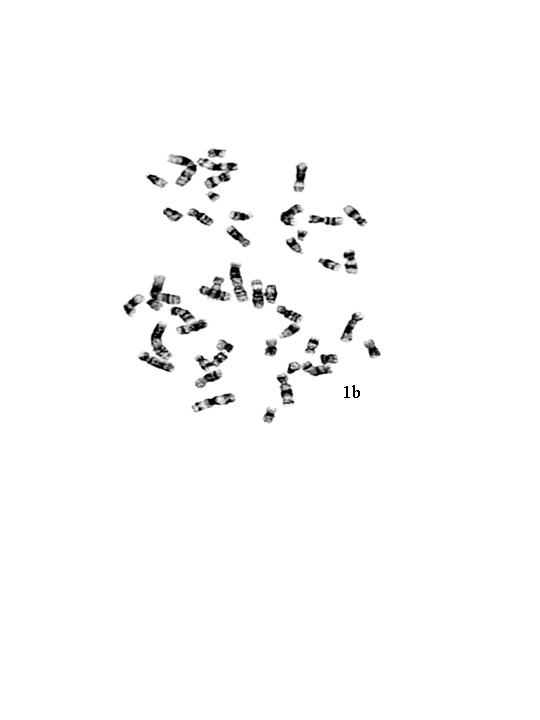
The gonadotrophin levels were markedly elevated, FSH levels were 70mIU/ml and LH level were 84mIU/ml (Normal: FSH 1.2-5mIU/ml; LH 2-9.8 mIU/ml). Chromosomal analysis performed on 50 G-banded metaphases showed 46, XX chromosomal complement (Figure 1 b & c). Fluorescent in situ hybridization (FISH) analysis was done using commercially available centromeric probes for chromosome X and Y (Vysis CEPX/CEPY) to rule out low level mosaicism. All the 200 cells scored showed XX complement (Figure 2a).
 |
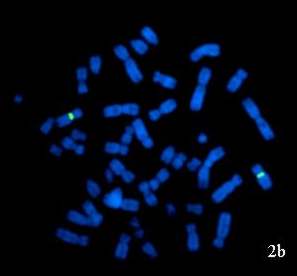 |
Figure 2: FISH images of interphase nuclei and metaphase chromosomes obtained from peripheral blood sample of 46,XX male patient: (a) Interphase nuclei and metaphase chromosomes hybridized with CEP X/CEP Y probe showing two red signals (chromosome X) representing XX constitution. (b) Metaphase chromosomes hybridized with LSI SRY/CEP X probe showing only two green signals (chromosome X) indicating the absence of SRY region. |
Further, FISH was done using probe specific for the sex determining region (SRY) (Vysis CEPX/LSI SRY) to identify any cryptic rearrangement resulting in translocation of SRY region on to the chromosome other than Y or to analyse the presence of SRY region even in absence of Y chromosome. A total of 200 metaphases and interphase cells were scored which revealed the absence of SRY region (Figure 2b).
DISCUSSION
XX males were recognized for the first time by de La Chapelle et al, in 1964
[8]. In 1966, Ferguson Smith [9] and his colleagues proposed that this condition
results from translocation of Y material including sex determining region (SRY)
on Y chromosome to the X chromosome. Berkovitz [10] and Vilain et al. [11] reported
46,XX males which were negative for SRY using PCR. McElreavey et al. [6] proposed
that SRY has been postulated to repress an inhibitor of male testicular development.
SRY negatively regulates an autosomal locus, Z, that switches off male determining
genes. In normal males SRY situated on the short arm of Y chromosome just proximal
to pseudoautosomal region I negatively regulates Z locus. It is possible that
in the present case XX male (SRY negative) may have a recessive mutation in
the Z locus, so that it cannot switch off the male determining genes [11] and
therefore the present case has normal male phenotypes and testicular development.
46,XX males lack the long arm of the Y chromosome (Yq). Which contains the Azoospermia
factor (AZF) loci and has gene complex critical for germ cell development and
differentiation [12]. Absence of AZF region in our case also explains for the
infertile phenotype and azoospermia. Numabe et al. [13] reported that 46,XX
males without Y sequences were hypospadiac but this was not seen in the present
case. FSH and LH levels were elevated in the present case as seen in cases with
severe testiculopathy and complete spermatogenic arrest leading to testicular
failure.
Thus, for the complete workup of XX males, it is important that conventional
cytogenetic analysis be followed by FISH analysis to determine the presence
of SRY gene. These investigations indicate that the amount of Y specific material
contributes to the phenotype heterogeneity. Inspite of the fact that SRY is
the testis determining factor (TDF), it is likely that other genes are also
involved in pathway of sex determination and differentiation process as both
upstream and downstream regulator genes.
ACKNOWLEDGEMENTS:
The authors are grateful to The Indian Council of Medical Research (ICMR), New Delhi for financial support, project no. 54/1/98.
REFERENCES
1. de la Chapelle A Nature and origin of males with XX sex chromosomes.
Am J Hum Genet 1972; 24:71-105.
2. Fechner PY, Marcantonio SM, Jaswaney V, Stetten G, Goodfellow PN, Migeon
CJ, Smith KD, Berkovitz GD, Amrhein JA, Bard PA, et al. The role of the sex-determining
region Y gene in the etiology of 46,XX maleness. J Clin Endocrinol Metab 1993;
76(3):690-695.
3. Kucheria K, Mohapatra I, Taneja N, Gupta DK, Ammini AC. Genetic heterogeneity
in true hermaphrodite. A report of two cases. J Reprod Med 1994; 39(7):550-552.
4. Eliana Ternes Pereira, JC Cabral de Almeida, A C Y R G Gunha, Michael Patton,
Rohan Taylor, Stephen Jeffery. Use of probes for ZFY, SRY and the Y pseudoautosomal
boundary in XX males, XX true hermaphrodites, and an XY female. J Med Genet
1991; 28:591-595.
5. de la Chapelle A Sex chromosomal abnormalities. In: Emery AE, Rimoin DE,
(eds) Principles and Practice of Medical Genetics. Churchill Livingstone. 1990;
Pp 273-299.
6. McElreavey K, Rappaport R, Vilain E, Abbas N, Richaud F, Lortat-Jacob S,
Berger R, LeConiat M, Boucekkine C, Kucheria K, Temtamy S, Nuhoul-Fekete C,
Brauner R, Marc Fellous. A minority of 46, XX true hermaphrodites are positive
for the Y-DNA sequence including SRY. Hum Genet 1992; 90:121-125.
7. WHO. WHO Laboratory Manual for the Examination of Human Semen and Semen and
Semen-Cervical Mucus Interaction. Cambridge, UK: Cambridge University Press
1999.
8. de la Chapelle A, Tippett PA, Wettestrand G, Page D. Genetic evidence of
X-Y interchange in human XX male. Nature 1984; 307:170-171.
9. Ferguson-Smith MA. X-Y chromosomal interchange in the aetiology of true hermaphroditism
and of XX Klinefelter's syndrome. Lancet 1966; 2(7461):475-476.
10. Berkovitz GD. Abnormalities of gonad determination and differentiation.
Sem Perinatology 1992; 16:289-298.
11. Vilain E, le Fiblec B, Morichon-Delvallez N, Brauner R, Dommergues M, Dumez
Y, Jaubet F, Boucekkine C, McElreavey K, Vekemans M, Fellous M. SRY-negative
XX fetus with complete male phenotype. Lancet 1994; 343(8891):240-241.
12. Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F,
Kohn FM, Schill WB, Farah S, Ramos HM, Castel A, Nieschlag E, Weidner W, Grone
H-J Jung A, Engel W, Haidl G. Human Y chromosome azoospermia factors (AZF) mapped
to different subregions in Yq 11. Hum Mol Genet 1996; 5:933-943.
13. Numabe H, Nagafuchi S, Nakahori Y, Tamura T, Kiuchi H, Namiki M, et al.
DNA analysi of XX and XX-hypospadiac males. Hum Genet 1990; 90:211-214.
First Published: September 2002
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-

