Browse through our Journals...
Fast Dissolving Tablet: A Novel Approach for Delivery of Carvedilol
Shailesh Sharma*, G.D. Gupta, C.P. Jain1, P.S. Naruka1
*Pharmaceutics Research Laboratory,
Department of Pharmaceutics
ASBASJS Memorial College of Pharmacy, Bela, Ropar, Punjab.
1Department of Pharmaceutics
B. N. College of Pharmacy, Udaipur, Rajasthan.
ABSTRACT
Carvedilol is a poorly water-soluble oral antihypertensive agent, with problems of variable bioavailability and bio-inequivalence related to its poor water-solubility. This work investigated the possibility of developing Carvedilol tablets, allowing fast, reproducible, and complete drug dissolution, by using drug solid dispersion in polyethylene glycol. Solubility studies were performed to investigate the drug-carrier interactions in solution, X-ray powder diffraction, were used to characterize the solid state of solid dispersions. The tablets were prepared by direct compression technique. The prepared tablets were evaluated for thickness, uniformity of weight, content uniformity, hardness, friability, wetting time, in vitro disintegration time and in vitro drug release. The tablets apart from fulfilling all official and other specifications, the Carvedilol dissolution profile from the newly developed tablets was clearly better than those from various conventional tablets at the same drug dosage. The stability studies conducted as per ICH guidelines at 40° and 75% RH showed insignificant loss in drug content and on physical evaluations at the end of six months.
KEYWORDS
Carvedilol, Fast Dissolving Tablet, Superdisintigrates, Solid dispersion, Enhance bioavailability.
INTRODUCTION
The concept of Fast dissolving Drug Delivery System emerged from the desire to provide patient with more conventional means of taking their medication. It is difficult for many patients to swallow tablets and hard gelatin capsules. Hence they do not comply with prescription, which results in high incidence of non-compliance and ineffective therapy1. In some cases such as motion sickness, sudden episodes of allergic attacks or coughing and unavailability of water, swallowing conventional tablets may be difficult2. Particularly the difficulty is experienced by pediatric and geriatric patients. Such problems can be resolved by means of Fast Dissolving Tablet. When put on tongue, this tablet disintegrates instantaneously, releasing the drug, which dissolves or disperses in the saliva. Some drugs are absorbed from the mouth, pharynx and esophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablet dosage form3. Carvedilol is both an alpha and a beta adrenoreceptor-blocking agent used in the treatment of various cardiovascular disorders such as angina pectoris, cardiac arrhythmia and hypertension. Its biological half-life (2.2 hours) is very short and it is 90% absorbed from GIT, but its bioavailability is only 10-20% indicating extensive first pass metabolism in liver. In view of substantial first pass effect and its shorter plasma half-life, therefore is an ideal drug candidate for rapid release drug delivery system.
MATERIALS AND METHODS
Materials
Carvedilol was obtained from Sun Pharma (Silvasa) Ac-Di-Sol, Crosspovidone, Xylitol, Sorbitol were obtained as gift samples from Signet Chemicals (Mumbai).
Methods
Preparation and Evaluation of Solid Dispersion
Solid dispersions were prepared with PEG 4000 in various ratios (1:1 to 1:8) by using melt fusion method. The fused mixtures were prepared by heating the corresponding ground mixture in a porcelain dish to about 50C above the melting point of PEG 4000 with continuous stirring for a minute. The samples were immediately quenched to 40C, the resulting solid was scraped out and stored in a desiccators at room temperature. The solid dispersions were evaluated for saturation solubility, drug content and X-Ray Diffraction studies.
Preparation and Evaluation of Tablet 4,5
Tablets were made from blends by direct compression method. All the ingredients (shown in Table 01) were passed through mesh no. 60. All the ingredients were co ground in a pestle motor. Finally talc and magnesium stereate were added and mixed for 5 minutes. The quality of tablet, once formulated by rule, is generally dictated by the quality of physicochemical properties of blends. The mixed blend of excipients was compressed using a single punch machine to produce convex faced tablets weighing 125 mg each with 2.85 mm thickness and 7.8 mm in diameter. The tablets were evaluated for General Appearance, Size and Shape, Uniformity of weight, Tablet hardness, Friability, Disintegration time, Wetting time, In vitro dispersion time, Content Uniformity.
RESULT AND DISCUSSION
The prepared solid dispersions were evaluated for drug content, saturation solubility and X Ray diffraction studies (Table 02).
Table 01: Formulation of Fast dissolving Tablet of Carvedilol
Ingredients |
FDT1 |
FDT2 |
FDT3 |
FDT 4 |
FDT 5 |
FDT 6 |
FDT 7 |
FDT 8 |
Carvedilol |
12.5 |
12.5 |
12.5 |
12.5 |
- |
- |
- |
- |
Carvedilol + PEG 4000(1:4) |
- |
- |
- |
- |
62.5 |
62.5 |
62.5 |
62.5 |
Ac Di Sol |
3.75 |
5 |
- |
- |
1.25 |
2.5 |
- |
- |
Crosspovidone |
- |
- |
3.75 |
5 |
- |
- |
1.25 |
2.5 |
Dextrose |
18 |
18 |
18 |
18 |
- |
- |
- |
- |
Lactose |
18 |
18 |
18 |
18 |
- |
- |
- |
- |
Sorbitol |
25 |
25 |
25 |
25 |
18.75 |
18.75 |
18.75 |
18.75 |
Xylitol |
19 |
17.75 |
19 |
17.75 |
12.5 |
11.25 |
12.5 |
11.25 |
Avicel pH 102 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
25 |
Talc |
1.25 |
1.25 |
1.25 |
1.25 |
2.5 |
2.5 |
2.5 |
2.5 |
Magnesium Stereate |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
Table 02: Evaluation of Solid Dispersion
Parameters |
Drug content (%) |
Saturation solubility (mg/ml) |
Ratio |
||
Pure Drug |
100 |
0.323±0.0215 |
1:1 |
99.91±1.302 |
23.341±1.257 |
1:2 |
98.61±1.294 |
24.837±1.072 |
1:3 |
97.25±0.416 |
26.359±0.986 |
1:4 |
99.92±0.256 |
29.957±0.549 |
1:5 |
99.25±0.419 |
29.998±1.024 |
1:6 |
97.94±1.253 |
30.001±1.002 |
1:7 |
98.26±1.035 |
28.098±0.458 |
1:8 |
99.65±0.983 |
32.214±1.914 |
Table 03 Evaluation of Fast Dissolving Tablets
Formulation |
FDT1 |
FDT2 |
FDT3 |
FDT4 |
FDT5 |
FDT6 |
FDT7 |
FDT8 |
Parameters |
||||||||
Bulk Density (gm/cm3) |
0.428 |
0.461 |
0.418 |
0.447 |
0.392 |
0.397 |
0.379 |
0.382 |
Tapped Density (gm/cm3) |
0.459 |
0.491 |
0.431 |
0.473 |
0.419 |
0.421 |
0.419 |
0.417 |
Hausners Ratio |
1.072 |
1.065 |
1.031 |
1.058 |
1.068 |
1.06 |
1.105 |
1.091 |
Compressibilty Index (%) |
7.242 |
6.507 |
3.11 |
5.816 |
6.887 |
6.045 |
8.554 |
9.162 |
Angle of Repose (O) |
25.34 |
24.42 |
26.34 |
28.24 |
27.66 |
29.47 |
29.98 |
28.64 |
Weight (mg) |
124.61±0.39 |
125.10±0.12 |
125.48± 0.19 |
124.89± 0.41 |
125.38± 0.87 |
125.54± 0.28 |
125.21± 0.18 |
126.25± 0.83 |
Hardness (kg/cm2) |
2.3±0.1 |
2.2±0.3 |
2.5±0.1 |
2.5±0.4 |
3.1±0.1 |
3.2±0.2 |
3.1±0.3 |
3.1±0.2 |
Friability (%) |
0.55± 0.23 |
0.62± 0.51 |
0.41±0.37 |
0.55±0.24 |
0.77±0.13 |
0.48±0.29 |
0.43±0.11 |
0.77±0.27 |
Disintegration Time (sec) |
28±2 |
21±4 |
31±2 |
24±5 |
54±4 |
42±6 |
60±3 |
44±5 |
Swelling Time (Sec) |
15±6 |
12±3 |
17±2 |
14±4 |
16±2 |
14±1 |
20±3 |
17±1 |
% Drug Release (5 min) |
90.671 |
92.063 |
77.789 |
85.323 |
97.042 |
99.266 |
90.693 |
94.948 |
Drug content from solid dispersion of Carvedilol:PEG 4000 (1:1 to 1:8) was found to be 97.94 % to 99.92%. It was observed that the saturation solubility of the drug was increased in (Sorenson’s Buffer pH 6.8) by converting the drug Carvedilol into solid dispersions which may be due to change in physical state of Carvedilol from crystalline to amorphous state, which was confirmed by XRD studies. The X- Ray diffraction pattern of solid dispersion of Carvedilol: PEG 4000 (1:4) showed no defined peak attributes to Carvedilol, this implies the absence of apparent crystalline in solid dispersion. However in the pure Carvedilol powder typical peak of Carvedilol was present, so confirming the satisfactory sensitivity of the method. The peaks are shown in Figure 01. On the basis of the obtained results the ratio of Carvedilol: PEG 4000 (1: 4) was optimized for further development of the fast dissolving tablet of Carvedilol.
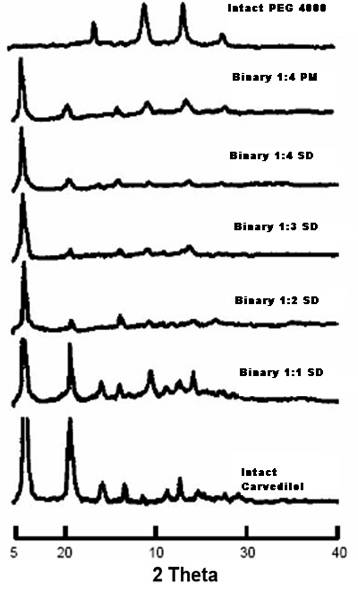
Figure 1 X-Ray Diffraction Spectrums





Figure 2 Different stages of Swelling of Fast Dissolving Tablet
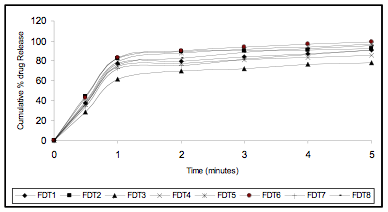
Figure 3 In Vitro Drug Release Profile (Zero Order Release)
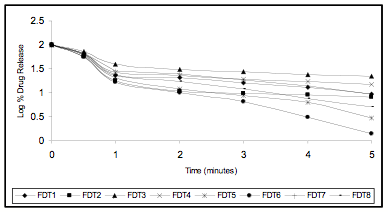
Figure 4 In Vitro Drug Release Profile (First Order Release)
All the tablets were exhibit in white color, odorless, convex in shape with smooth surface with zero defects. The average weight of the prepared tablet was found 123.11 to 126.68 mg. The thickness and diameter of the tablet was found 2.85 mm, 7.8 mm respectively. A tablet requires certain amount of hardness to withstand the mechanical shocks in handling, packaging and at the time of application. The hardness of the prepared tablet varied from 2.1 to 3.3 Kg/cm2 which have satisfactory strength to withstand the mechanical shocks.
The friability of all the formulation was found to be less than 1.0 %. The results shows resistance to loss of weight indicates the tablet’s ability to withstand abrasion in handling, packaging and shipment. The disintegration time of the tablets was varied form 19 to 60 seconds. The tablets with Ac-Di-Sol may disintegrate faster then the tablets with the crosspovidone. The in vitro swelling time of all the formulations were varied between 09 to 20seconds. The drug content of all the formulations was varied from 12.293 to 12.600 mg per tablet. The correlation of variation was found to be less than 0.054 %, indicating uniformity of the drug content in the prepared tablets. The release found to be at the end of five minutes 77.789 - 96.063% by using of superdisintegrates. The formulations with Ac – Di- Sol shows more release than the tablets with Crosspovidone. An increase (4 -5%) in the drug release was observed when the drug used as solid dispersion with PEG 4000 in ratio of 1:4 with low concentration of disintegrates (1 and 2%). From the in-vitro drug release profile it is evident that the kinetics of drug release is first order for all the prepared fast dissolving tablets as the plot between log percent drug retained versus time showed good linearity. The coefficient of determination of R2 values much closer to 1. The good relationship was evidenced in the Hixon – Crowell's Cube Root Law which signifies the drug is assumed to dissolve out from matrix or from surface of the device. All the formulations showed no significant variation in all the parameters under the test period. The drug degradation was found to follow first order kinetics the data obtained from accelerated stability studies, when fitted to the Arrhenius studies, the shelf life of prepared tablet was found to be 520 to 832 days.
CONCLUSION
The prepared tablet gives benefit in terms of patient compliance, low dosing, rapid onset of action, increased bio-availability, low side effect and good stability which make these tablets popular as a dosage form for the treatment of hypertension.
REFERENCES
1. Seager H. Drug-deliver products and the zydis fast-dissolving dosage form. J. Pharm. and Pharmacol. 1998; 50: 375-382.
2. Habib W, Khankari R, Hontz J. Fast-dissolving drug delivery systems. Critical Reviews Therapeutic Drug Carrier Systems. 2000; 17(1): 61-72.
3. Corveleyn S, Remon, J P. Formulation and production of rapid disintegrating tablets by lyophilization using hydrochlorthiazide as a model drug. Int. J. Pharm. 1997; 152: 215-225.
4. Ringard J, Guyot-Hermann AM, Calculation of disintegrant critical concentration in order to optimize tablets disintegration. Drug Dev. Ind. Pharm. 1997; 14 (15-17): 2321-2339.
5. Bi, Y. et al. (1996) Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem. Pharm. Bull. 44 (11), 2121-2127.
ACKNOWLEDGEMENT
Author(s) is thankful to Sun Pharmaceuticals, Silvasa for providing Carvedilol, and for Signet Chemicals, Mumbai for providing drug, polymer and other ingredients.
Copyright © Priory Lodge Education Limited
First Published June 2007
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


