Xin-sheng Deng M.D. Victoria J. Simpson M.D., Ph.D. and Richard A. Deitrich Ph.D.
Department of Pharmacology, University of Colorado Health Sciences Center, Denver,
Colorado, USA 80262
Key Words: Propofol; nitric oxide; antioxidant; anesthetic.
Corresponding author: Xin-sheng Deng
Propofol, an intravenous anesthetic, is similar in chemical structure to the active nucleus of antioxidant substances such as alpha-tocopherol (vitamin E), butylhydroxytoluene and acetylsalicylic acid (aspirin). Recent studies have demonstrated that some effects of propofol may lie in its antioxidant properties and the likely involvement of nitric oxide. This review article focuses on the relationship between nitric oxide and propofol. There is an implication that nitric oxide is responsible for the hemodynamic responses of propofol. The antioxidant effect of propofol may also extend its anesthetic application.
Propofol is a rapidly acting intravenous anesthetic agent that has gained widespread acceptance for anesthesia and sedation. The rapid and complete recovery profile associated with propofol offers advantages over other injectable anesthetic agents (1,2). Administration of propofol produces pronounced hemodynamic responses, particularly the decrease in arterial blood pressure (3-11). Published reports have not agreed as to the mechanism of propofol-mediated hypotension, but some investigators have attributed significant decrease in systemic vascular resistance to the blood vessel-dilation property of propofol to (12,13) with the likely involvement of endothelium-derived relaxing factor (nitric oxide) (14). Most importantly, propofol has been found to bear antioxidant capacity due to its structural similarity to -tocopherol (15-43), which is strongly related to free radicals. At this point propofol is not only an anesthetic agent but also an antioxidant drug. In this review, we have focused on published literature relating to free radical nitric oxide and its oxidative reaction with propofol.
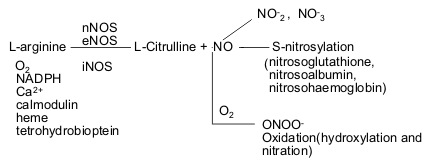
Formation, decomposition and reactivity of nitric oxide
Nitric oxide (NO) is an
inorganic gas synthesized by the enzyme nitric oxide synthase (NOS) in which
amino acid L-arginine is oxidized to NO and equal amount of L-citrulline(44-46).
Three NOS isoforms have been identified: neuronal NOS (nNOS),
endothelial NOS (eNOS), and iNOS (47). The former two isozymes are constitutively
expressed (constitutive NOS) and have physiologic roles; the last is usually
present only after the induction by inflammatory stimuli.
Figure One: Nitric oxide formation, decomposition, transportation and reaction.

The genes encoding eNOS, nNOS and iNOS have been cloned and sequenced (47-49). These isoenzymes are distinct and located on different chromosomes (7, 12 and 17, respectively). They are structurally related to the cytochrome p-450 supergene family and consist of a single polypeptide chain containing L-arginine, heme and calmodulin binding sites as well as a complete NADPH diaphorase. Required co-factors include oxygen, NADPH, calcium (constitutive NOS) and tetrahydrobiopterin (50, 51).
Neuronal NOS (nNOS) is mainly distributed in the nervous system. NO from nNOS functions as a neurotransmitter (52-58) in long-term potentiation (59), gonadotropin secretion (60-64), sexual behavior (65-69), regulates emotional behaviors (70, 71) and autonomic outflow to the cardiovascular system (72,73). nNOS is also present in the kidney (74), skeletal muscle (75-77) and myocardium (78), as well as pancreatic islets (79, 80).
Endothelial NOS is predominately present in the endothelium of blood vessels. NO released from endothelium is found as endothelium-derived relaxing factor, or EDRF, which modulates vascular tone and accommodates change in blood flow (81-84). Endothelial NOS is also present in some immune cells (85, 86). Accordingly, eNOS 'knockout" mice are routinely hypertensive (87-93).
Inducible NOS is expressed in tissues of the immune system (macrophages, leukocytes and other phagocytic cells) on stimulation with cytokines and/or endotoxin (94-96), vascular smooth muscle (94, 97), endothelium (97, 98), kidney (mesangium, tubules) (99,100) and other sites (pancreas, liver, enterocytes, airway, pneumocytes) (101-113). NO from iNOS is present in a large amount. It exerts antimicrobial, cytotoxic effects and immunoregulation (cytokine production, apoptosis and signalling) in the immune system. Therefore, inducible NOS knockout mice exhibit loss of immune function and minor hypotension (113-121).
NO functions by diffusion to kill bacteria and other microbial pathogens (122-124)
or possibly acts at the enzyme guanylyl cyclase levels and augmenting cyclic
guanosine monophosphate (cGMP) production (125-129). Compared with neurotransmitter
receptors or related adenylyl and guanylyl cyclases (130,131), the NO receptor
enzyme appears rather unremarkable. It is composed of two different subunits,
but only two isoforms have been shown to exist at the protein level: the 11
isoform, which is expressed widely, and the 21 isoform present in human placenta
(132-134). Several receptor systems, including N-methyl-D-aspartate (NMDA) (135,
136), muscarinic (137, 138), and gamma-aminobutyric acid ( -GABA) receptors
(139, 140) and A2-adrenoceptors (141), have been shown to mediate their action
via the NO-cGMP pathway.
NO is a labile species with a half-life of only a few seconds in biological systems. It degrades rapidly to NO2- (nitrite). Nitrite is unstable and it is further converted to the end product NO3- (nitrate). Putative intermediate metabolites include an array of low and high molecular weight thiols--nitrosoglutathione, nitrosoalbumin, S-nitrosohaemoglobin (142). This is not only a mechanism for scavenging NO but also serves to transport NO and is the molecular basis for biological effects in its own right. In the presence of O2, NO reacts with O2- to form ONOO- (peroxynitrite) and other NO radicals as well. Overproduction of NO can lead to cytotoxicity. NO rapidly oxidizes sulfhydryl groups and thioethers in peptide, proteins and lipids (143). In addition, NO nitrates and hydroxylates aromatic compounds, including guanosine (DNA damage) (144), benzene (145, 146)), tyrosine (147), tryptophan (148), 4-hydroxyphenylacetic acid (149), and -tocopherol (150). These deleterious effects of peroxynitrite may disturb cell-signalling processes (Fig. 1).
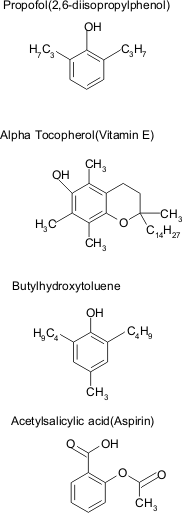
Structural formulae of propofol, alpha tocopherol, butylhydroxytoluene and acetylsalicylic acid. There is a hydroxyl subsitituent and a benzene group in all four compounds.
Propofol and its clinical relevance with NO
Propofol (2,6-diisopropylphenol) is an intravenous anesthetic that is widely used for both induction and maintenance of general anesthesia. The pharmacokinetics of propofol is best described by a three-compartment model: the central compartment, the shallow peripheral compartment and the deep peripheral compartment. Of the greatest importance is the rapid clearance of propofol (rapid and complete recovery), which is approximately ten times faster than that of thiopental. This made propofol the best controllable intravenous hypnotic from a pharmacokinetic point of view (1,2, 151) Its clinical uses include ambulatory anesthesia, monitored anesthesia care, neuroanesthesia, cardiac anesthesia, pediatric anesthesia and sedation in the intensive care unit (2).
Use of propofol for induction of anesthesia causes decrease in arterial pressure and systemic vascular resistance. Systolic arterial pressure (SAP) is decreased after the start of induction ; diastolic pressure (DAP) is decreased at 60 s after start of induction and further decreases are seen until 210 s after induction (6). The precise mechanism(s) of propofol-induced hypotension is not known. Many studies have attributed the hypotensive responses to decreases in peripheral resistance. This can be prevented by effective volume loading (152), but cannot be attenuated by administration of a fluid preload (10). Induction of anesthesia with an opioid-benzodiazepine combination followed by a maintenance infusion of propofol, supplemented with an inhalational agent or opioid analgesic or both, appears to control blood pressure as well (2). Some studies suggested that propofol-mediated hypotension is due in part to an inhibition of the sympathetic nervous system (153) and to an impairment of baroreflex mechanism (154). A reduction in plasma norepinephrine concentrations after propofol has been demonstrated also (155). Recently, a possible involvement of endothelium-derived relaxing factor or nitric oxide was proposed in the rapid onset of vasodilatation produced by propofol (14). It was reported that propofol stimulated nitric oxide release from cultured porcine aortic endothelia cells and an inhibitor of NO blocked the effects of propofol (156). In a different study, propofol showed a contractile effect in isolated aortas from spontaneously hypertensive rats in the present of a nitric oxide inhibitor (157). However, another study examined the effects of propofol on rat aortic and pulmonary artery rings and demonstrated a marked relaxation, which was endothelium-independent (158). In addition, the same effect was observed in isolated mesenteric arteries from humans (159). Further studies on the mechanism responsible for the reduction in systemic vascular resistance and hypotension of propofol are needed.
Antioxidant activity of propofol
Free radicals are believed to contribute the tissue injuries associated with many pathological processes such as ischemia, tissue anoxia, inflammatory process, infection, carcinogenesis, neurodegenerative disorder and diabetes (160-162). In such diseases, antioxidants can protect tissues by inhibiting lipid peroxide formation or increasing the activity of the glutathione antioxidant system, among other mechanisms (34, 163).
Propofol has a structure (2,6.diisopropylphenol) similar to that of known antioxidants (Fig. 2), such as tocopherol (vitamin E), acetylsalicylic acid and butylhydroxytoluene (16, 17, 34, 164).
The ability of propofol to inhibit the formation of lipid peroxides has been found in several media in which free radicals are produced, e.g., liver and brain microsomes in the rat (16), liver mitochondria in the rat (18, 23), and chemical media enriched in arachidonic acid or linoleic acid (17, 25). Using normal rat tissues (36) and an in vitro model of cerebral anoxia in the rat, it was found that the antioxidant effect of propofol is manifested not only as an inhibition of lipid peroxidation, but also as a decrease in tissue consumption of glutathione (34).
Studies in animals show that propofol, indeed, reduces the formation of lipid peroxides (16, 18, 23, 34, 36). In humans, there was no effect on plasma lipid peroxide levels in patients given propofol (22). However, others showed an increase in plasma antioxidant capacity in patients anesthetized with propofol (31, 33). The highest levels of peroxides occur in cell membranes, rather than in plasma, and the antioxidant glutathione pathway is an important intracellular antioxidant system. In a group of surgical patients who were given propofol anesthesia, propofol showed antioxidant effects as evidenced by the inhibition of lipid peroxidase production in the platelet membrane and changes in the glutathione antioxidant system (42). In other experiments, propofol enhanced red blood cell antioxidant capacity in swine and humans (32).
Propofol, like other phenol-based antioxidant compounds, also acts directly
as a free radical scavenger. Studies on the ameliorating effect of propofol
in inhibiting radical production revealed that it preferentially scavenges organoradical
species. In aqueous
suspension it is more efficient than butylated hydroxy-toluene (BHT) as a free
radical scavenger of riboflavin radicals and in blocking formation of malondialdehyde
degradation products generated from lipid hydroperoxides of arachidonic acid
(20). In additional experiments it was found, using electron spin resonance
(ESR), that propofol reacted with oxygen free radicals or peroxynitrite to form
phenoxyl radical (17, 165). Moreover, it was demonstrated employing mass spectrometry,
that propofol could react with NO to generate nitro-propofol in vitro (forming
phenoxyl radical) (166). Thus, propofol is a peroxynitrite scavenger. Because
of these reactions, propofol has neuroprotective properties against injuries
caused by ischemia/reoxygenation (34, 167-169). Also, propofol prevents and
reverses the inhibition of excitatory amino acid uptake in astrocytes exposed
to tert-butyl hydroperoxide. The ability of propofol to defend against peroxide-induced
inhibition of glutamate clearance may prevent the pathologic increase in extracellular
glutamate at synapses, and thus delay or prevent the onset of excitotoxic neuronal
death (40, 170). Furthermore, propofol had a protective effect in neurons against
acute mechanical injury (171). A water-soluble prodrug of propofol protects
from neuronal cell death from oxidative injury caused by glutamate (43). This
is consistent with the clinical observation that use of propofol is associated
with significant cerebral protection. The same protection was obtained in heart
reperfusion injury. Isolated perfused Wistar rat hearts were subjected to either
warm global ischaemia (Langendorff) or cold St. Thomas' cardioplegia (working
heart mode) in the presence or absence of propofol. It was found that with the
presence of propofol the heart injuries were significantly less, probably as
a result of diminished oxidative stress (172). In isolated, working rat hearts
subjected to ischemia, followed by reperfusion, it was observed that propofol
attenuated mechanical dysfunction, metabolic derangement, and lipid peroxidation
during reperfusion (24, 173). Additional experiments demonstrated that in vivo,
propofol ameliorated dysfunction of the myocardium but not of the coronary endothelium
resulting from brief ischaemia and reperfusion. The protection may be related,
at least in part, to its ability to reduce lipid peroxidation (174).
However, the antioxidant properties of propofol are different depending on the formulation of propofol. Propofol inhibited the chemiluminescence (CL, a measure of oxidative stress) produced by stimulated polymorphonuclear (PMN) leukocytes in a dose dependent manner (until 5 x 10 -5 M, a clinically relevant concentration), while Diprivan (the commercial form of propofol) and intralipid (IL, vehicle solution of PPF in Diprivan, composition: 1.2% egg phosphatide, 2.25% glycerol) were not dose-dependent inhibitors. The CL produced by endothelial cells was dose-dependently inhibited by Diprivan and PPF, and weakly by IL (not dose-dependent). In cell free systems, dose-dependent inhibitions were obtained for the three products with a lower effect for IL. Diprivan efficaciously protected endothelial cells submitted to an oxidant stress, while IL was ineffective. By HPLC, it was demonstrated that PPF was not incorporated into the cells. The drug thus acted by scavenging the active oxygen species released into the extracellular medium. IL acted in the same manner, but was a less powerful antioxidant (38).
In conclusion, there is an implication that propofol enhances NO production in vascular system and that NO is probably responsible for the hypertension. The unique antioxidant ability and free radical scavenger of propofol may lead to further broaden its current clinical application in the future.
1. Cockshott ID .Propofol ('Diprivan') pharmacokinetics and metabolism--an overview. Postgrad Med J. 1985;61 Suppl 3:45-50. Review
2. Smith I, White PF, Nathanson M, Gouldson R. Propofol. An update on its clinical use. Anesthesiology. 1994;81:1005-43. Review.
3. Grounds RM, Twigley AJ, Carli F, et al. The hemodynamic effects of intravenous induction. Comparison of the effects of thiopentothal and propofol. Anaesthesia. 1985;40:735-40.
4. Muzi M, Berens RA, Kampine JP, Ebert TJ. Venodilation contributes to propofol-mediated hypotension in humans. Anesth Analg. 1992;74:877-83.
5. Ebert TJ, Muzi M, Berens R, et al. Anesthesiology. 1992;76:725-33.
6. Lindgren L, Yli-Hankala A, Randell T, et al. Haemodynamic and catecholamine responses to induction of anaesthesia and tracheal intubation: comparison between propofol and thiopentothal. Br J Anaesth. 1993;70:306-10.
7. Robinson BJ, Buyck HC, Galletly DC. Effect of propofol on heart rate, arterial pressure and digital plethysmograph variability. Br J Anaesth. 1994;73:167-73.
8. el-Beheiry H, Kim J, Milne B, Seegobin R. Prophylaxis against the systemic hypotension induced by propofol during rapid-sequence intubation. Can J Anaesth. 1995;42:875-8.
9. Robinson BJ, Ebert TJ, O'Brien TJ, et al. Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997;86:64-72.
10. Turner RJ, Gatt SP, Kam PC, et al. Administration of a crystalloid fluid preload does not prevent the decrease in arterial blood pressure after induction of anaesthesia with propofol and fentanyl. Br J Anaesth. 1998;80:737-41.
11. Hertzog JH, Campbell JK, Dalton HJ, Hauser GJ. Propofol anesthesia for invasive procedures in ambulatory and hospitalized children: experience in the pediatric intensive care unit. Pediatrics. 1999;103:E30.
12. Boer F, Ros P, Bovill JG, et al. Effect of propofol on peripheral vascular resistance during cardiopulmonary bypass. Br J Anaesth. 1990;65:184-9
13 Rouby JJ, Andreev A, Leger P, et al. Peripheral vascular effects of thiopental and propofol in humans with artificial hearts. Anesthesiology. 1991;75:32-42
14. Editorial. Yin and yang in vasomotor control. Lancet. 1988;2(8601):19-20. Review
15. Eriksson O. Effects of the general anaesthetic Propofol on the Ca2(+)-induced permeabilization of rat liver mitochondria. FEBS Lett. 1991;279:45-8
16. Musacchio E, Rizzoli V, Bianchi M, et al. Antioxidant action of propofol on liver microsomes, mitochondria and brain synaptosomes in the rat. Pharmacol Toxicol. 1991;69:75-7.
17. Murphy PG, Myers DS, Davies MJ, et al. The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth. 1992;68:613-8.
18. Eriksson O, Pollesello P, Saris NE. Inhibition of lipid peroxidation in isolated rat liver mitochondria by the general anaesthetic propofol. Biochem Pharmacol. 1992;44:391-3.
19. Kvam C, Granese D, Flaibani A, et al. Hyaluronan can be protected from free-radical depolymerisation by 2,6-diisopropylphenol, a novel radical scavenger. Biochem Biophys Res Commun. 1993;193:927-33.
20 Green TR, Bennett SR, Nelson VM. Specificity and properties of propofol as an antioxidant free radical scavenger. Toxicol Appl Pharmacol. 1994;129:163-9.
21. Cervantes M, Ruelas R, Chavez-Carrillo I, et al. Effects of propofol on alterations of multineuronal activity of limbic and mesencephalic structures and neurological deficit elicited by acute global cerebral ischemia. Arch Med Res. 1995;26:385-95.
22. Khinev S, Dafinova K, Tenchova V, Bakalova R. The lipid peroxidation level and antioxidant status of the plasma in patients operated on under propofol (Diprivan) anesthesia. Khirurgiia (Sofiia). 1995;48:23-5.
23. Aarts L, van der Hee R, Dekker I, et al. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995;357:83-5
24. Kokita N, Hara A. Propofol attenuates hydrogen peroxide-induced mechanical and metabolic derangements in the isolated rat heart. Anesthesiology. 1996;84:117-27.
25. Hans P, Deby C, Deby-Dupont G, et al. Effect of propofol on in vitro lipid peroxidation induced by different free radical generating systems: a comparison with vitamin E. J Neurosurg Anesthesiol. 1996;8:154-8.
26. Salgo MG, Pryor WA. Trolox inhibits peroxynitrite-mediated oxidative stress and apoptosis in rat thymocytes. Arch Biochem Biophys. 1996;333:482-8.
27. Kahraman S, Demiryurek AT. Propofol is a peroxynitrite scavenger. Anesth Analg. 1997;84:1127-9.
28. Miele M, Fillenz M. Changes in extracellular brain ascorbate in rat striatum in response to administration of non-volatile anaesthetic agents. Br J Anaesth. 1997;78:588-90.
29. Kelicen P, Ismailoglu UB, Erdemli O, Sahin-Erdemli I. The effect of propofol and thiopentone on impairment by reactive oxygen species of endothelium-dependent relaxation in rat aortic rings. Eur J Anaesthesiol. 1997;14:310-5.
30. Young Y, Menon DK, Tisavipat N, et al. Propofol neuroprotection in a rat model of ischaemia reperfusion injury. Eur J Anaesthesiol. 1997;14:320-6.
31. Hans P, Deby-Dupont G, Deby C, et al. Increase in antioxidant capacity of plasma during propofol anesthesia. J Neurosurg Anesthesiol. 1997;9:234-6.
32. Ansley DM, Lee J, Godin DV, et al. Propofol enhances red cell antioxidant capacity in swine and humans.Can J Anaesth. 1998;45:233-9.
33. Stratford N, Murphy P. Antioxidant activity of propofol in blood from anaesthetized patients. Eur J Anaesthesiol. 1998;15:158-60.
34. De La Cruz JP, Villalobos MA, Sedeno G, Sanchez De La Cuesta F. Effect of propofol on oxidative stress in an in vitro model of anoxia-reoxygenation in the rat brain. Brain Res. 1998;800:136-44.
35. Mouithys-Mickalad A, Hans P, Deby-Dupont G, et al. Biochem Biophys Res Commun. 1998;249:833-7.
36. De La Cruz JP, Sedeno G, Carmona JA, Sanchez de la Cuesta F. The in vitro effects of propofol on tissular oxidative stress in the rat. Anesth Analg. 1998;87:1141-6.
37. Reber A, Huber PR, Ummenhofer W, et al. General anaesthesia for surgery can influence circulating melatonin during daylight hours. Acta Anaesthesiol Scand. 1998;42:1050-6.
38. Mathy-Hartert M, Deby-Dupont G, Hans P, et al. Protective activity of propofol, Diprivan and intralipid against active oxygen species. Mediators Inflamm. 1998;7:327-33.
39. Bao YP, Williamson G, Tew D, et al. Antioxidant effects of propofol in human hepatic microsomes: concentration effects and clinical relevance. Br J Anaesth. 1998;81:584-9.
40. Sitar SM, Hanifi-Moghaddam P, Gelb A, et al. Propofol prevents peroxide-induced inhibition of glutamate transport in cultured astrocytes. Anesthesiology. 1999;90:1446-53.
41. Ansley DM, Sun J, Visser WA, et al. High dose propofol enhances red cell antioxidant capacity during CPB in humans. Can J Anaesth. 1999;46:641-8.
42. De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg. 1999;89:1050-5.
43. Sagara Y, Hendler S, Khoh-Reiter S, et al. Propofol hemisuccinate protects neuronal cells from oxidative injury. J Neurochem. 1999;73:2524-30.
44. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664-6.
45. Schmidt HH, Nau H, Wittfoht W, et al. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988;154:213-6.
46 Kelm M, Feelisch M, Spahr R, et al. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988;154:236-44.
47. Forstermann U, Schmidt HH, Pollock JS, et al. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991;42:1849-57. Review
48. Moncada S, Higgs EA, Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361-74. Review.
49. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-42. Review.
50. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298 ( Pt 2):249-58. Review.
51. White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627-31.
52. Kadowaki K, Kishimoto J, Leng G, Emson PC. Up-regulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamo-hypophysial system after chronic salt loading: evidence of a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994;134:1011-7.
53. Huang Z, Huang PL, Panahian N, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883-5.
54. Nelson RJ, Demas GE, Huang PL, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383-6.
55. Schulz JB, Matthews RT, Jenkins BG, et al. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo.J Neurosci. 1995;15:8419-29.
56. Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179-90. Review.
57. Kiss JP, Sershen H, Lajtha A, Vizi ES. Inhibition of neuronal nitric oxide synthase potentiates the dimethylphenylpiperazinium-evoked carrier-mediated release of noradrenaline from rat hippocampal slices. Neurosci Lett. 1996;215:115-8.
58. Dawson TM, Dawson VL. Nitric oxide synthase: role as a transmitter/mediator in the brain and endocrine system. Annu Rev Med. 1996;47:219-27. Review.
59. Altememi GF, Alkadhi KA. Nitric oxide is required for the maintenance but not initiation of ganglionic long-term potentiation. Neuroscience. 1999;94:897-902.
60. Gonzalez D, Aguilar E. In vitro, nitric oxide (NO) stimulates LH secretion and partially prevents the inhibitory effect of dopamine on PRL release. J Endocrinol Invest. 1999;22:772-80.
61. Honaramooz A, Cook SJ, Beard AP, et al. Nitric oxide regulation of gonadotrophin secretion in prepubertal heifers1. J Neuroendocrinol. 1999;11:667-76.
62. Pinilla L, Tena-Sempere M, Gonzalez D, Aguilar E. The role of nitric oxide in the control of basal and LHRH-stimulated LH secretion. J Endocrinol Invest. 1999;22:340-8.
63. Gobbetti A, Zerani M. In vitro, nitric oxide (NO) stimulates LH secretion and partially prevents the inhibitory effect of dopamine on PRL release. J Endocrinol Invest. 1999;22:772-80.
64. Adams ML, Nock B, Truong R, Cicero TJ. Nitric oxide control of steroidogenesis: endocrine effects of NG-nitro-L-arginine and comparisons to alcohol. Life Sci. 1992;50:PL35-40.
65. Pfaus JG. Neurobiology of sexual behavior. Curr Opin Neurobiol. 1999; 9:751-8. Review.
66. Mani SK, Allen JM, Rettori V, et al. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci U S A. 1994;91:6468-72.
67. Hull EM, Lumley LA, Matuszewich L, et al. The roles of nitric oxide in sexual function of male rats. Neuropharmacology. 1994;33:1499-504.
68. McCann SM, Kimura M, Karanth S, et al. Nitric oxide controls the hypothalamic-pituitary response to cytokines. Neuroimmunomodulation. 1997;4:98-106. Review.
69. Hull EM, Lorrain DS, Du J, et al. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105-16. Review.
70. Persoons JH, Schornagel K, Breve J, et al. Acute stress affects cytokines and nitric oxide production by alveolar macrophages differently. Am J Respir Crit Care Med. 1995;152:619-24.
71. Bugajski J. Acute stress affects cytokines and nitric oxide production by alveolar macrophages differently. Am J Respir Crit Care Med. 1995;152:619-24.
72. Umans JG, Levi R. Nitric oxide in the regulation of blood flow and arterial pressure. Annu Rev Physiol. 1995;57:771-90. Review.
73. Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin Sci (Colch). 1999;97:5-17. Review.
74. Wilcox CS, Welch WJ. Macula densa nitric oxide synthase: expression, regulation, and function. Kidney Int Suppl. 1998;67:S53-7.
75. Chao DS, Hwang PM, Huang F, Bredt DS. Localization of neuronal nitric oxide synthase. Methods Enzymol. 1996;268:488-96.
76. Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271:11204-8.
77. Wang Y, Newton DC, Marsden PA. Neuronal NOS: gene structure, mRNA diversity, and functional relevance. Crit Rev Neurobiol. 1999;13:21-43. Review.
78. Ursell PC, Mayes M.
Anatomic distribution of nitric oxide synthase in the heart.
Int J Cardiol. 1995;50:217-23. Review.
79. Worl J, Wiesand M, Mayer B, et al. Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in rat and human pancreas: influence of fixation. Histochemistry. 1994;102:353-64.
80. Mensah-Brown EP, Bailey TA, Pallot DJ, Garner A. Peptidergic hormones and neuropeptides, and aminergic neurotransmitters of the pancreatic islets of the Houbara bustard. J Anat. 2000;196 (Pt 2):233-41.
81. Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708-16.
82. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524-6.
83. Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. 1999;31:577-96. Review.
84. Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879-86. Review.
85. Furuke K, Burd PR, Horvath-Arcidiacono JA, et al. Human NK cells express endothelial nitric oxide synthase, and nitric oxide protects them from activation-induced cell death by regulating expression of TNF-alpha. J Immunol. 1999;163:1473-80.
86. Ishiuchi N, Yoshino S, Yokoyama M, Asano G. Expression of endothelial nitric oxide synthase and inducible nitric oxide synthase in synovium of rheumatoid arthritis. Ryumachi. 1999;39:749-56.
87. Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176-81.
88. Rudic RD, Shesely EG, Maeda N, et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731-6.
89. Fagan KA, Fouty BW, Tyler RC, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103:291-9.
90. Stauss HM, Godecke A, Mrowka R, et al. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33:1359-63.
91. Sun D, Huang A, Smith CJ, Stackpole CJ, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288-93.
92. Huang PL. Neuronal and
endothelial nitric oxide synthase gene knockout mice.
Braz J Med Biol Res. 1999;32:1353-9. Review.
93. Heeringa P, van Goor H, Itoh-Lindstrom Y, et al. Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2000;156:879-88.
94. Vanhoutte PM. Inducible nitric oxide synthase and vascular smooth muscle.
Jpn J Pharmacol. 1992;58 Suppl 2:192P-199P. Review.
95. Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993;54:171-8. Review
96. Cook HT, Bune AJ, Jansen AS, et al. Cellular localization of inducible nitric oxide synthase in experimental endotoxic shock in the rat. Clin Sci (Colch). 1994;87:179-86.
97. Schini VB, Catovsky S, Scott-Burden T, Vanhoutte PM. The inducible nitric oxide synthase is impaired by thrombin in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1992;20 Suppl 12:S142-4.
98. Jensen BO, Holmsen H. From endothelium-derived relaxing factor to L-arginine and nitrogen monoxide. Tidsskr Nor Laegeforen. 1992;112:2531-5. Review.
99. Markewitz BA, Michael JR, Kohan DE. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993;91:2138-43.
100. Ahn KY, Mohaupt MG, Madsen KM, Kone BC. In situ hybridization localization of mRNA encoding inducible nitric oxide synthase in rat kidney. Am J Physiol. 1994;267(5 Pt 2):F748-57.
101. Corbett JA, Wang JL, Misko TP, et al. Nitric oxide mediates IL-1 beta-induced islet dysfunction and destruction: prevention by dexamethasone. Autoimmunity. 1993;15:145-53.
102. Corbett JA, Kwon G, Turk J, McDaniel ML. IL-1 beta induces the coexpression of both nitric oxide synthase and cyclooxygenase by islets of Langerhans: activation of cyclooxygenase by nitric oxide.Biochemistry. 1993;32:13767-70.
103. Eizirik DL, Leijerstam F. The inducible form of nitric oxide synthase (iNOS) in insulin-producing cells. Diabete Metab. 1994;20:116-22. Review.
104. Geller DA, Lowenstein CJ, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993;90:3491-5.
105. Di Silvio M, Geller DA, Gross SS, et al. Inducible nitric oxide synthase activity in hepatocytes is dependent on the coinduction of tetrahydrobiopterin synthesis. Adv Exp Med Biol. 1993;338:305-8.
106. Geller DA, Nussler AK, Di Silvio M, et al. Cytokines, endotoxin, and glucocorticoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc Natl Acad Sci U S A. 1993;90:522-6.
107. Sorrells DL, Friend C, Koltuksuz U, et al. Inhibition of nitric oxide with aminoguanidine reduces bacterial translocation after endotoxin. challenge in vivo. Arch Surg. 1996;131:1155-63.
108. ter Steege J, Buurman W, Arends JW, Forget P. Presence of inducible nitric oxide synthase, nitrotyrosine, CD68, and CD14 in the small intestine in celiac disease. Lab Invest. 1997;77:29-36.
109. Robbins RA, Springall DR, Warren JB, et al. Inducible nitric oxide synthase
is increased in murine lung epithelial cells by cytokine stimulation. Biochem
Biophys Res Commun. 1994;198:835-43.
111. Berisha HI, Pakbaz H, Absood A, Said SI. Nitric oxide as a mediator of oxidant lung injury due to paraquat. Proc Natl Acad Sci U S A. 1994;91:7445-9.
112. Nijkamp FP, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther. 1995;329:81-96. Review.
113. Liu S, Adcock IM, Old RW, et al. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1993;196:1208-13.
114. MacMicking JD, North RJ, LaCourse R, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94:5243-8.
115. Gilkeson GS, Mudgett JS, Seldin MF, et al. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997;186:365-73.
116. Mishima S, Xu D, Lu Q, Deitch EA. Bacterial translocation is inhibited in inducible nitric oxide synthase knockout mice after endotoxin challenge but not in a model of bacterial overgrowth. Arch Surg. 1997;132:1190-5.
117. Iadecola C, Zhang F, Casey R, et al. Delayed reduction of ischemic brain qinjury and neurological deficits in mice lacking the inducible nitric oxide synthase gene.J Neurosci. 1997;17:9157-64.
118. Fenyk-Melody JE, Garrison AE, Brunnert SR, et al. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940-6.
119. Gunnett CA, Chu Y, Heistad DD, et al. Vascular effects of LPS in mice deficient in expression of the gene for inducible nitric oxide synthase. Am J Physiol. 1998;275(2 Pt 2):H416-21.
120. Hom GJ, Grant SK, Wolfe G, et al. Lipopolysaccharide-induced hypotension and vascular hyporeactivity in the rat: tissue analysis of nitric oxide synthase mRNA and protein expression in the presence and absence of dexamethasone, NG-monomethyl-L-arginine or indomethacin. J Pharmacol Exp Ther. 1995;272:452-9.
121. MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641-50.
122. Anderson JH. The metabolism of hydroxylamine to nitrite by Nitrosomonas. Biochem J. 1964;91:8-17.
123. Russell C. The effect
of nitric oxide on the growth of Escherichia coli M.
Experientia. 1965;21:625.
124. Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65-70. Review.
125. Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39:163-96. Review.
126 Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203-7.
128. Mittal CK, Murad F.
Properties and oxidative regulation of guanylate cyclase.
J Cyclic Nucleotide Res. 1977;3:381-91. Review.
129. Martin E, Davis K, Bian K, et al. Cellular signaling with nitric oxide and cyclic guanosine monophosphate. Semin Perinatol. 2000;24:2-6. Review.
130. Drewett JG, Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 1994;15:135-62. Review.
131. Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461-80. Review.
132. Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997;18:484-91. Review.
133. Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334-50. Review.
134. Russwurm M, Behrends S, Harteneck C, Koesling D. Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem J. 1998;335 ( Pt 1):125-30.
135. Amir S. Blocking NMDA receptors or nitric oxide production disrupts light transmission to the suprachiasmatic nucleus. Brain Res. 1992;586:336-9.
136. Kawamata T, Omote K. Activation of spinal N-methyl-D-aspartate receptors stimulates a nitric oxide/cyclic guanosine 3,5-monophosphate/glutamate release cascade in nociceptive signaling. Anesthesiology. 1999;91:1415-24.
137. Zhuo M, Meller ST, Gebhart GF. Endogenous nitric oxide is required for tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1993;54:71-8.
138. Sales ME, Espanol AJ, Sterin-Borda L, et al. Protein kinase C regulates NO-cGMP pathway in muscarinic receptor activation by HIV+-IgA. Int J Mol Med. 1999;3:633-7.
139. Fedele E, Conti A, Raiteri M. The glutamate receptor/NO/cyclic GMP pathway in the hippocampus of freely moving rats: modulation by cyclothiazide, interaction with GABA and the behavioural consequences. Neuropharmacology. 1997;36:1393-403.
140. Lin Q, Wu J, Peng YB, et al. Nitric oxide-mediated spinal disinhibition contributes to the sensitization of primate spinothalamic tract neurons. J Neurophysiol. 1999;81:1086-94.
141. Martire M, Pistritto G, Mores N, et al. Presynaptic A2-adrenoceptors and neuropeptide Y Y2 receptors inhibit [3H]noradrenaline release from rat hypothalamic synaptosomes via different mechanisms. Neurosci Lett. 1995;188:9-12.
142. Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control.Nature. 1996;380:221-6.
143. Szabo C. DNA strand breakage and activation of poly-ADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. Free Radic Biol Med. 1996;21:855-69. Review.
144. Yermilov V, Rubio J, Becchi M, et al. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045-50.
145. Knight JA. Radiolysis of nitrobenzene. Radiat Res. 1972;52:17-24.
146. Holder JW. Nitrobenzene carcinogenicity in animals and human hazard evaluation. Toxicol Ind Health. 1999;15:445-57. Review.
147. van der Vliet A, Eiserich JP, Shigenaga MK, Cross CE. Reactive nitrogen species and tyrosine nitration in the respiratory tract: epiphenomena or a pathobiologic mechanism of disease? Am J Respir Crit Care Med. 1999;160:1-9. Review.
148. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469-73. Review.
149. van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617-25.
150. Arroyo PL, Hatch-Pigott V, Mower HF, Cooney RV. Mutagenicity of nitric oxide and its inhibition by antioxidants. Mutat Res. 1992;281:193-202.
151 Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92:727-38.
152. Goodchild CS, Serrao JM. Cardiovascular effects of propofol in the anaesthetized dog. Br J Anaesth. 1989;63:87-92.
153. Hoka S, Yamaura K, Takenaka T, Takahashi S. Propofol-induced increase in
vascular capacitance is due to inhibition of sympathetic vasoconstrictive activity.
Anesthesiology. 1998;89:1495-500.
154. Cullen PM, Turtle M, Prys-Roberts C, et al. Effect of propofol anesthesia on baroreflex activity in humans. Anesth Analg. 1987;66:1115-20.
155. Valtonen M, Iisalo E, Kanto J, Rosenberg P. Propofol as an induction agent in children: pain on injection and pharmacokinetics. Acta Anaesthesiol Scand. 1989;33:152-5.
156. Petros AJ, Bogle RG, Pearson JD. Propofol stimulates nitric oxide release from cultured porcine aortic endothelial cells. Br J Pharmacol. 1993;109:6-7.
157. Boillot A, Laurant P, Berthelot A, Barale F. Effects of propofol on vascular reactivity in isolated aortae from normotensive and spontaneously hypertensive rats. Br J Anaesth. 1999;83:622-9.
158. Park WK, Lynch C 3d, Johns RA. Effects of propofol and thiopental in isolated rat aorta and pulmonary artery. Anesthesiology. 1992;77:956-63.
159. Moreno L, Martinez-Cuesta MA, Muedra V, et al. Role of the endothelium in the relaxation induced by propofol and thiopental in isolated arteries from man. J Pharm Pharmacol. 1997;49:430-2.
160. Balliwell B, Gutteridge
JMC. Free Dadicals in Biology and Medicine, 2nd ed. Oxford: Clarendon Press,
1989.
161. Tamir S, Tannenbaum SR. The role of nitric oxide (NO.) in the carcinogenic
process. Biochim Biophys Acta. 1996;1288:F31-6. Review.
162. Webster NR, Nunn JF. Molecular structure of free radicals and their importance in biological reactions. Br J Anaesth. 1988;60:98-108. Review.
163. Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335-58. Review.
164. Grootveld M, Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo.Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986;237:499-504.
165. Mouithys-Mickalad A, Hans P, Deby-Dupont G, et al. Propofol reacts with peroxynitrite to form a phenoxyl radical: demonstration by electron spin resonance. Biochem Biophys Res Commun. 1998;249:833-7.
166. Cudic M, Ducrocq C. Transformations of 2,6-diisopropylphenol by NO-derived nitrogen oxides, particularly peroxynitrite. Nitric Oxide. 2000 Apr;4(2):147-56.
167. Ito H, Watanabe Y, Isshiki A, Uchino H. Neuroprotective properties of propofol and midazolam, but not pentobarbital, on neuronal damage induced by forebrain ischemia, based on the GABAA receptors. Acta Anaesthesiol Scand. 1999;43:153-62.
168. Yamasaki T, Nakakimura K, Matsumoto M, et al. Effects of graded suppression of the EEG with propofol on the neurological outcome following incomplete cerebral ischaemia in rats. Eur J Anaesthesiol. 1999;16:320-9.
169. Yamaguchi S, Midorikawa Y, Okuda Y, Kitajima T. Propofol prevents delayed neuronal death following transient forebrain ischemia in gerbils. Can J Anaesth. 1999;46:593-8.
170. Hans P, Bonhomme V, Collette J, et al. Propofol protects cultured rat hippocampal
neurons against N-methyl-D-aspartate receptor-mediated glutamate toxicity. J
Neurosurg Anesthesiol. 1994;6:249-53.
171. Hollrigel GS, Toth K, Soltesz I. Neuroprotection by propofol in acute mechanical injury: role of GABAergic inhibition. J Neurophysiol. 1996;76:2412-22.
172. Javadov SA, Lim KH,
Kerr PM, et al. Protection of hearts from reperfusion injury by propofol is
associated with inhibition of the mitochondrial permeability transition. Cardiovasc
Res. 2000;45:360-9.
173. Ko SH, Yu CW, Lee SK, et al. Propofol attenuates ischemia-reperfusion injury
in the isolated rat heart. Anesth Analg. 1997;85:719-24.
174. Yoo KY, Yang SY, Lee J, et al. Intracoronary propofol attenuates myocardial but not coronary endothelial dysfunction after brief ischaemia and reperfusion in dogs.Br J Anaesth. 1999;82:90-6.
First Published: September 2003
All pages copyright ©Priory Lodge Education Ltd 1994-2004.
.