Author : Walter Tarello, veterinary surgeon,
C.P. 42, 06061 Castiglione del Lago
PERUGIA (Italy)
Email
the author
Abstract Introduction Methods Results Discussion Conclusions References Figures
The aim of this study was to compare
the concentration and serological tests results in two groups of 7 dogs each,
with heartworm infection, and living in endemic and non-endemic areas. All the
14 dogs had microfilariae of Dirofilaria immitis in the blood, detected
by a filtration test (Difil-test). Differently, serological results were always
negative in dogs living in non-endemic area and in 2 out of 7 dogs from endemic-area.
These results suggest that false seronegativity seems easy to occur in no or
low-endemic areas. Consequently, the search of microfilariae by Knott or filtration
tests is still strongly recommended in such places , preferably combined with
serological tests.
At the present time, heartworm disease caused by the filarial helminth Dirofilaria
immitis is not endemic in most of the European countries (Greeve et al., 1983;
Schrey, 1996). All cases reported in UK, the Netherlands, Sweden, Switzerland,
Austria, and Germany, have been imported previously from endemic areas (Schrey,
1996; Zahler et al., 1997; Bucklar et al., 1998). In Germany, canine dirofilariasis
has been discovered on routine blood tests for microfilaria in 13% of dogs with
history of travel to Italy, Portugal, Spain and Corsica and in 10% of dogs imported
from Italy, Spain and Portugal (Schrey, 1996).
During the last 20 years the infestation has spread to colder areas where
suitable vectors exist, such as Bretagne in France (Doby et al., 1986), Switzerland
(Bucklar et al., 1998) and northern provinces in Italy (Genchi et al. 1995;
Rossi et al., 1996). The absence of large number of suitable vectors in
the UK probably explains why the disease has not yet become endemic in the country,
despite importation of infected dogs (Murdock, 1984).
In Italy, endemic areas are the Po plane and the Tuscan coast (Fig. 5), meanwhile
the remaining parts of the country are considered free or low endemic areas
(Genchi et al., 1995; Rossi et al., 1996). Introduction of the disease to such
areas is thought to have resulted initially from the free movement of infected
animals from warmer, higher incidence areas. Diagnosis of canine dirofilariosis
in animals which originate from a low-endemic region is difficult for 3 main
reasons:
1) possible absence of circulating microfilariae (occult disease),
2) high frequency of a low number of adults, which in turn produces false negative
results, using either ELISA or latex agglutination test,
3) possible false sero-positive results, due to cross-reaction with adult antigens
of Dirofilaria (Nochtiella) repens (Schrey, 1996), the agent of subcutaneous
dirofilariasis causing chronic pruritic dermatitis in dogs (Tarello , 1999).
It is generally thought that concentration tests (Knott, filtration) are the most sensitive, because detection of microfilaria in the blood is diagnostic for the presence of adults in the pulmonary arteries (BSAVA, 1998). This observation is particularly true for low-endemic or newly colonized areas , where a low number of adults (false seronegatives) and/or a hight prevalence of Dirofilaria repens (false seropositives) are to be observed (Genchi et al., 1995).
A negative concentration test, does not however, rule out a diagnosis of dirofilariosis
because reports say that 20 to 30 per cent of canine infections and the majority
of infected cats are negative for microfilaria (Davoust and Ducos de Lahitte,
1989; Rawlings and McCall, 1982; Rawlings and Calvert, 1995). Consequently,
occult disease can be diagnosed by tests using monoclonal antibodies to circulating
adult antigens (ELISA or latex agglutination).
The first aim of this study was comparing the concentration and serological
tests results, obtained from 2 samples of dogs with heartworm patent infection,
in an endemic (Alexandria, Piedmont) and a non-endemic area (Fermo, Marche)
of Italy (Fig. 5).
The second aim was that of determining how correlated are the two different
test techniques in not endemic areas, where suspected cases are not often encountered
and familiar to the clinicians, their diagnosis is much more difficult on objective
grounds, and missing cases can contribute to spreading the infestation among
dogs, in association with lack of preventive medication.
Animals
Fourteen adult dogs from two different areas and with a diagnosis of heartworm
disease, were examined on the basis of laboratory tests results for Dirofilaria
immitis infection. Seven symptomatic canine dirofilariasis were collected in
1998, during a 8-months period (spring to autumn) of clinical practice in highly
endemic area (Alessandria, Piedmont; Fig. 5). The apparently low number of registered
cases is justifyied by the widespread use of prophylaxis (Ivermectine, Milbemycin
oxime) in that region. The other seven patent cases of heartworm disease were
collected in 1999 during a 8-months period (spring to autumn) of practice in
a non-endemic area (Fermo, Ascoli Piceno province) of Italy, where prophylaxis
is fairly rare (Fig. 5).
Protocol
No selection was made in admitting recorded cases to the present study, but
the physical examination had to reveal at least one
symptom of dirofilariasis : cough, weight loss, weakness (BSAVA, 1998). Two
specimens (2+2 ml.), an whole blood sample in EDTA and a serum sample, were
collected from each dog, and submitted to concentration test (Difil-test) and
to canine heartworm antigen test (Wittness Dirofilaria, Merial). The samples
for the differential identification of microfilariae were fixed using 2 per
cent formalin, filtered and examined by light microscopy at x10 and x40 magnifications.
The 7 samples from the non-endemic area (Fermo) were subsequently tested for
the heartworm antigens using PetCheck (IDEXX Laboratories) and Wittness Dirofilaria
(Merial).
No routine clinical haematology tests or radiographic abnormalities are diagnostics
for dirofilariasis (Rawlings and Calvert, 1995). Thereby these examinations
were not considered. Medical treatment followed any diagnosis of heartworm disease.
Microfilariae of D. immitis (1 to 9 per sample) were detected in all the seven
dogs with patent heartworm disease living in the highly endemic area of Alessandria
(Piedmont). Serological results were positive in 5 out of 7 animals.
In the non-endemic area of Fermo (Ascoli Piceno province), concentration test
results were similar and microfilaraemia due to D. immitis (1 to 8 per sample)
was present in the total number of dogs with clinical manifestations (Fig.1,2,4),
without significant quantitative differences in comparison with the results
coming from the endemic area. Unexpectedly, in contrast with these microscopical
findings, serological results in this group of dogs were always negative , using
subsequently both PetChek and Wittness Dirofilaria tests for canine heartworm
antigen detection .
It is interesting to note that animals so tested were all symptomatic: coughing
was the principal sign (13/14), followed by poor appetite (5/14), vomiting (3/14),
weight loss (3/14), weakness (2/14) and collapse (1/14).
No particular differences in severity and duration of symptoms could be detected
between the two canine groups. Complete resolution followed medical therapy
(melarsomine dihydrochloride, aspirin, ivermectin) in 6 out of 7 dogs from the
endemic area. One 13-years-old mixed male, with high number of microfilariae
and antigens-positive, died during the therapy.
Comparatively, 5 dogs from the low endemic area experienced a complete remission
after identical treatment, meanwhile 2 dogs died.
Comparison between direct identification of microfilariae and canine heartworm
antigen tests in two groups of 7 + 7 symptomatics dogs has confirmed that concentrations
tests (Difil-test) are the most sensitive and, furthermore, that they appear
particularly useful in non-
endemic or newly colonized areas. As a matter of fact, none of seven dogs from
a non-endemic area in Central Italy (Fermo, Marche), with D. immitis microfilariae
(fig. 1,2,4) in the blood and patent disease, proved seropositive with two antigen
tests (PetChek and Wittness). Differently, in the endemic area of North Italy
(Alexandria, Piedmont), 5 out of 7 microfilaraemic and symptomatics dogs resulted
seropositive (Witness).
Although the number of cases here reported is limited and statistical analysis
is not possible, the results of the present study show that difficulties in
heartworm diagnosis in non-endemic area may be due not only to the unfamiliarity
of clinicians with the disease but, also, to false sero-negative diagnostic
test results, apparently due to low number of adults nematodes carried by every
infected dog.
The frequency with which this occurs, as a consequence also of increasing pet's
travel, deserve further large-scale investigations.
In a recent study from another non-endemic area (Munchen, Germany), among 72
imported or travelling dogs with D. immitis microfilariae in the blood, and
examined between 1993-96, 27 (37.5%) resulted sero-negative with the ELISA test
(Zahler et al., 1997), apparently confirming the results here reported.
Two are the others canine filariases releasing microfilariae in the blood and
described in Italy: Dirofilaria (Nochtiella) repens and Acanthocheilonema (syn.:
Dipetalonema) reconditum (Pampiglione et al., 1995; Tarello, 1999).
Differential identification is made by light microscopy (x10,x40) using the
concentration tests (Knott's test, Difil-test). When fixed using 2 per cent
formalin, microfilariae of D. immitis are 262-338 mm in length and 4-6,2 mm
in width (Schrey, 1996), distinguishing them from the non-pathogenic filarial
larvae of A. reconditum which are shorter and thinner ( 241-287 mm x 3,8-5 mm).
Furthermore, the tails of A. reconditum microfilariae are frequently curved
as a ovariectomy hook (Fig. 3). Larvae of Dirofilaria (Nochtiella) repens are
the greatest, 270-379 mm in lenth and 6-8,3 mm in width (Pampiglione et al.,1995),
with a characteristic cephalic end roundish, short and clear (Fig.6). Comparatively,
the cephalic end of D. immitis microfilariae is cone-shaped and dark (Fig.1,2)
and their tail is very thin (Fig.4).
Detection of D. immitis microfilariae in a seronegative dog, can occur when
only a single gravid female lives in the pulmonary arteries of the host . In
fact, according to diagnostics manufacturers, infestations due to males, non-gravid
females or less than two gravid females are difficult to identify by serological
tests and can produce false negativities (Schrey, 1996).
To which extent this happen in practice has never been properly investigated.
The results of this preliminary study on a small number of animals naturally
infected , indicate that false seronegativity seems easy to occur in non-endemic
areas, which represents the major parts of italian and european territories.
This observation suggests that the search for microfilariae must not be abandoned,
and preferably coupled with serological tests, which are important tools in
enzootic regions, providing semiquantitative data upon the parasitic burden
of infection (Rawlings and Calvert, 1995). Combining the Knott test and an antigen
test improves overall accuracy. For instances, Hoover et. al. (1996) obtained
an accuracy of 95% when both the Knott test and the DiroCheck antigen test were
used.
Geographic distribution of D. immitis and D. repens coincides (Fig. 5) in many
areas of South Europe (Genchi et al., 1995; Rossi et al., 1996) and cross reactivity
between the two nematodes is regularly shown by different tests, producing false
seropositive results (Schrey, 1996). To prevent diagnostic errors, a concentration
test must be performed even in such cases.
Occult disease, frequently reported at a rate of 20 to 30 per cent (Rawlings
and McCall, 1982; Davoust and Ducos de Lahitte, 1989), was never observed during
the 2-years period of the present work. Occult dirofilariosis is a particular
aspect of large-scale epidemiological and routine screenings and its rate is
largely dependant on non-objective factors, such as individual skills and experience
in microfilariae detection. Furthermore, dogs included in this study for examinations
were all symptomatics and this might well explain the percentage of microfilaraemia
recorded and the absence of occult disease.
In summary, serological tests for heartworm disease are frequently negative
in non-endemic areas because of the low number of D. immitis adults in the pulmonary
arteries of the host. The presence of a single female and/or one to many males
is hardly detected by these tests. Apparently, this condition is common in non-enzootic
areas.
In such cases, accurate concentrations tests, however, can demonstrate circulating
microfilariae in many dogs, avoiding wrong diagnosis and diffusion of dirofilariasis
to newly colonized areas.
BSAVA's Scientific Committee. (1998) Heartworm disease. Journal of Small Animal Practice. 39, 407-408.
Bucklar H, Scheu U, Mossi R, Deplazes P. (1998) Is dirofilariosis in dogs spreading in south Switzerland ? Schweiz Arch Tierheilkd. 140, 255-60
Davoust B, Ducos de Lahitte. (1989) Evolution de l'enzootie de dirofilariose dans les chenils militaires du sud est. Revue de Médecine Vétérinaire.140, 15-19.
Doby JM, Guiguen C, Lefevre R. (1986) Présence de Dirofilaria immitis (Leidy, 1856) chez le chien en Bretagne. Bulletin Société Francaise de Parasitologie. 4, 51-54.
Genchi C, Venco L, Magnino B, Di Sacco B, Perera L, Bandi C, Pignatelli P, Formaggini L, Mazzucchelli M. (1993) Aggiornamento epidemiologico sulla filariosi del cane e del gatto. Veterinaria. 2, suppl., 5-11.
Genchi C, Solari Bazano F, Bandi C, Di Sacco B, Venco L, Vezzoni A, Cancrini G. (1995) Factors influencing the spread of heartworm in Italy; interaction between Dirofilaria immitis and Dirofilaria repens . In: Proceedings of the Heartworm Symposium '95 . Soll M.D., Knight D. II, eds., American Heartworm Society, Batavia, IL, 65-71.
Greeve RB, Lok JB, Glickman LT. (1983) Epidemiology of canine heartworm infection. Epidemiology Revue. 5, 220-246.
Hoover JP, Campbell GA, Fox JC, Claypoo PL, Mullins SB. (1996) Comparison of eight diagnostic blood tests for heartworm infection in dogs. Canine Practice. 21, 11-24.
Murdoch DB. (1984) Heartworm in the United Kingdom. Journal of Small Animal Practice. 25, 299-305.
Pampiglione S, Canestri Trotti G, Rivasi F. (1995) Human dirofilariasis due to Dirofilaria (Nochtiella) repens : a review of world literature. Parassitologia. 37 :149-193.
Rawlings CA, Mc Call JW. (1982) Four types of occult Dirofilaria immitis infections in dogs. Journal of American Veterinary Medical Association. 180, 1323-1326.
Rawlings CA, Calvert CA. (1995) Heartworm
disease. In: E.S.J. Ettinger ESJ and Feldman EC. (4th ed.): Textbook of Veterinary
Internal Medicine, W.B. Saunders, Philadelphia, 1046-1068.
Rossi L, Pollono F, Meneguz PG, Gribaudo L, Balbo T. (1996) An epidemiological
study on canine filarioses in north-west Italy: what has changed in 25 years
? Veterinary Research Communications. 20, 308-315.
Schrey CF. (1996) Epidemiologische Fallanalyse der kardiovaskularen Dirofilariose (Herzwurmerkrankung) bei Hunden in Deutschland. Dissertation for the degree of Doctor of Veterinary Medicine, der Freien Universitat Berlin.
Tarello W. (1999) La dirofilariose sous-cutanée à Dirofilaria (Nochtiella) repens chez le chien. Revue bibliographique et cas clinique. Revue de Médecine Vétérinaire. 150, 691-702.
Zahler M, Glaser B, Gothe R. (1997)
Imported parasites in dogs: Dirofilaria repens and Dipetalonema recoditum. Tierarztlische
Praxis. 25, 388-92.
1) Dirofilaria immitis microfilaria (x10, filtration test) in the blood of a
seronegative symptomatic dog from the non-endemic area of Fermo (Marche, Italy)
.
2) Dirofilaria immitis microfilaria (x4) in a fresh blood smear from a seronegative
dog , living in non-endemic area (Fermo, Italy).

3) Detail of the caudal end of a Achantocheilonema reconditum microfilaria (x40,
filtration test).

4) Detail of the caudal end of a Dirofilaria immitis microfilaria (x40, filtration
test), from a seronegative and symptomatic dog living in non-endemic area (Fermo,
Marche, Italy).
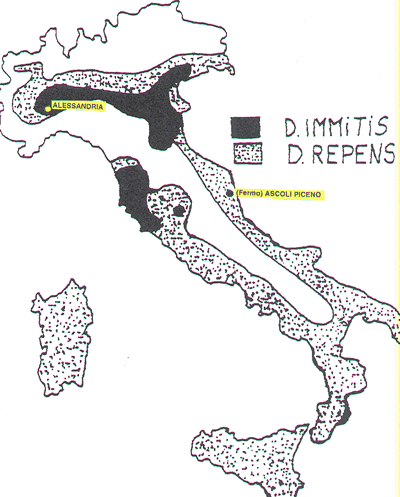
5) Map of Italy, showing the endemic areas for Dirofilaria immitis (black) and
for Dirofilaria (Nochtiella) repens (dots) and the towns of Alessandria and
Fermo (Ascoli Piceno province).
6) Dirofilaria (Nochtiella) repens microfilaria (x10), the causative agent of
subcutaneous dirorilariasis, associated with pruritic dermatitis in dogs.
All pages copyright ©Priory Lodge Education Ltd 1994-2004.
First Published 2/01/2001