A Case Report
*Department of Biochemistry, University of Texas Health Center at Tyler, Tyler, Texas, USA^Equine Medical Center & Veterinary Hospital, Gresham, Texas, USA**Coloproctology Center, Takano Hospital, Kumamoto, Japan
Summary![]() Introduction
Introduction![]() Report
Report![]() Results
Results![]() Discussion
Discussion ![]() Conclusions
Conclusions![]() References
References ![]() Table
One
Table
One ![]() Table
Two
Table
Two ![]() Previous
Page
Previous
Page
Feline fibrosarcoma are highly malignant tumours and are known to develop at vaccination sites. In this study we report the treatment of a case of feline fibrosarcoma with a combination of intradermal administration of lipopolysaccharide from Pantoea agglomerans (LPSp) and oral cyclophosphamide. While this regime has been successfully used in the treatment of human tumours, we believe that this is its first use in a cat. The patient was treated for five months, and during this time we showed prolonged stimulation of plasma TNFa activity following drug administration. The cat apparently remained tumour free during the treatment period and we believe that this combination therapy may prove useful in the treatment of this particularly aggressive and invasive tumour.
Fibrosarcoma remains an intractable and devastating disease both in human and veterinary medicine. In the domestic cat, fibrosarcoma has now been associated with the use of vaccines and the development of these tumours appears to occur in around 0.02% of vaccine injections (Hendrick et al, 1994). Once diagnosed, the prognosis remains guarded to poor. Even with repeated surgeries and/or surgery with radiation therapy the expected tumour free period is only 4.5 to 5 months and the reported survival times range from 9 to 16 months (Davidson et al, 1997).
Some 100 years ago, Coley noted the therapeutic efficacy of a mixed bacterial vaccine "Coley's Toxin" on human cancer (Coley, 1883). The active component of Coley's Toxin was found to be lipopolysaccharide (LPS) (Shear & Turner, 1943). Recently, a low molecular mass (5kDa) LPS from Pantoea agglomerans (LPSp) has been purified and used successfully in the treatment of human tumours (Goto et al, 1996). The antitumour effects of LPSp are mediated by the induction of TNFa (Inagawa et al, 1997), and are augmented by chemotherapeutic agents such as cyclophosphamide (Inagawa et al, 1997, Inagawa et al, 1998).
Here we report the treatment of a feline fibrosarcoma using primary surgical excision followed by intradermal administration of LPSp in conjunction with oral cyclophosphamide.
The cat "Tinker", a seven-year-old, 5.1 kg, spayed, female, domestic shorthair, which had been regularly vaccinated for rabies, feleuk, and feline rhinotracheitis/panleukopenia/Calici was presented for annual vaccinations and routine dental work. At that time, a large mass approximately 25mm in diameter was noted in the inter-scapular region. The mass was firm and attached to the underlying tissue. Serosanguinous fluid was aspirated from the lump, and the cytology at this time revealed a predominance of polymorphonuclear cells and macrophages. To exclude a possible infection at the injection site, the cat was put on a regimen of oral Clavamox liquid (62.5mg b.i.d.) for two weeks. However, during the first week of treatment, the mass had returned to its previous size, and remained so until being drained at the end of the fourth week. At this time, 20 ml of fluid was removed, which only partially deflated the mass. Surgery was performed and an underlying tumour was excised. Tissues were submitted to the Texas Veterinary Medical Diagnostic Lab for histopathological analysis. The histology, in which the tumour was graded as previously described (Davidson, 1997), revealed a high-grade fibrosarcoma in the subcutis. At several sites, tumour cells were noted to extend into the borders of the examined tissue, and total excision of the tumour could not be confirmed. At this point, the prognosis was guarded.
The possibility of treating Tinker with a combination of LPSp and cyclophosphamide was discussed with the owners, and informed consent obtained. Two weeks post operative the cat was put on a regimen of cyclophosphamide (25mg three times per week, orally) in conjunction with intradermal injections of LPSp twice per week. Since the tolerance of the cat for LPSp was unknown, an initial dose of 600ng/kg was used and increased over a 2 week period to 10,000ng/kg (0.05mg LPSp in 0.2ml saline). The initial dose of LPSp was based on the amount administered to humans (Goto et al, 1996), and extrapolated for the size of the cat. In each case the LPSp was administered id, twice per week on the dorsum of the cat at a site approximately 5cm from the original surgical site. The site was chosen to minimize any adverse effects on the wound healing. Approximately 2-4 hr after each administration of LPSp at the higher dose there was a single episode of food vomiting. However, the appetite remained strong, and no other side effects were noted. Treatment at the higher dosage was continued for a total of 23 weeks. TNFa was measured using a bioactivity assay utilizing the WEHI 164 cell line, as previously described (Khabar et al, 1995).
Day 0: Baseline physical examination, CBC and blood chemistries were performed (two weeks post operative) just prior to initiation of treatment. The baseline and pre- and post-LPSp hematological results are shown in Table 1. The body weight and temperature at each examination are shown in Table 2.
Physical examination on day 0 revealed a scar in the interscapular space from the recent removal of the fibrosarcoma tumour. The surgical site was healing well, with no palpable masses and there were no other abnormal findings. Blood chemistries were all within normal range.
Day 49: There were no abnormal findings on physical examination. CBC was normal
Day 98: There were no abnormal findings on physical examination. Blood samples were taken immediately prior to id LPSp administration and post treatment samples was taken after 3, 6 hours and 24 hrs. The hematological findings are given in Table 2. Since TNFa is believed to mediate the anti-tumour effects of LPSp treatment, we measured TNFa in the plasma using WEHI mouse fibrosarcoma cell line (Khabar, 1995). The TNFa activity in the plasma after LPSp administration was approximately two and a half times higher than in the pre-treatment sample and remained elevated at 24hr. (Figure 1)Day 173: Treatment was stopped. The cat was obviously thinner, but active and bright. Physical examination revealed the presence of a small mass (5mm x 5mm) palpable just posterior to the previous surgical site. CBC was normal.
Day 214: The cat had not received treatment over the previous month. She remained very bright and alert and had gained weight, and the CBC was normal. The mass posterior to the previous surgical site now measured approximately 10mm x 5mm, and a decision was made to explore the site surgically.
Day 217: Following induction with xylazine and ketamine im, the previous surgical site and the area with the palpable mass just posterior to it were explored surgically and the tissues removed en bloc. The area where the mass had been felt was found to be grossly similar to a lipoma and no other clearly defined mass was found. Histopathology was performed on the resected tissues and several sections of a previously biopsied mass were examined. The section approximately 40mm posterior to the original surgical site contained a mass consisting of sheets of haphazardly arranged spindle cells having abundant fibrillar eosinophilic cytoplasm. The nuclei of the cells were oval and vesicular. Mitotic figures were fairly common. Furthermore, there was evidence of moderate, diffuse edema and congestion. The surgical margins appeared clean and the prior surgical site did not contain any neoplasia. There was some granulation tissue found and some inflammation associated with suture material was noted. The findings are consistent with fibrosarcoma posterior to the original surgical site and fibroplasia and inflammation at the original surgical site.
Following surgical excision of the feline fibrosarcoma and treatment with a combination of intradermal LPSp and oral cyclophosphamide, the cat remained healthy and tolerated the treatment well, with only mild discomfort. The cat, apparently, remained tumour free during the treatment period, and it is uncertain whether the newly identified neoplasms were present at the time of the original surgery, and have remained suppressed by the treatment or if they are new neoplasms which may have developed after the cessation of treatment. However, the possibility that such a treatment can inhibit the growth of feline fibrosarcoma is intriguing. Further studies are required, but we believe that this combination therapy may prove useful in the treatment of this particularly aggressive and invasive tumour.
Coley WB. (1883) A preliminary note on the treatment of inoperable sarcoma by the toxic product of Erysipelas. Post-graduate. 8:278-286
Davidson EB, Gregory CR, Kass PH. (1997) Surgical excision of soft tissue fibrosarcomas in cats. Veterinary Surgery. 26: 265-269
Goto S, Sakai S, Kera J, et al. (1996) Intradermal administration of lipopolysaccharide in treatment of human cancer. Cancer Immunology, Immunotherapy. 42: 255‑261
Inagawa H, Nishizawa T, Takagi K, et al. (1997) Antitumour mechanism of intradermal administration of lipopolysaccharide. Anticancer Research 17: 1961‑1964
Inagawa H, Ohshiro S, Nishizawa T, Goto S, Soma G-I, Mizuno D'I. (1997) Augmentation of antitumour effect of endogenously induced tumour necrosis factor by cyclophosphamide. Anticancer Research. 17: 55-60
Inagawa H, Nishizawa T, Honda T, et al. (1998) Mechanisms by which chemotherapeutic agents augment the antitumour effects of tumour necrosis factor: involvement of the pattern shift of cytokines from Th2 to Th1 in tumour lesions. Anticancer Research 18:3957‑64
Hendrick MJ, Shofer FS, Goldschmidt MH et al. (1994) Comparison of fibrosarcomas that developed at vaccination sites and at nonvaccination sites in cats: 239 cases (1991‑1992). Journal of the
American Veterinary Medical Association. 205: 1425‑1429
Iwamoto I, Goto S, Kera J, et al. (1996) Mechanistic analysis of high antitumour effect of intradermal aministration of lipopolysaccharide from Panoea Agglomerans. Medical Oncology. 13: 103‑109
Khabar KS, Siddiqui S, Armstrong JA. (1995) WEHI‑13VAR: a stable and sensitive variant of WEHI 164 clone 13 fibrosarcoma for tumour necrosis factor bioassay. Immunology Letters 46:107‑110
Otto CM, Rawlings CA. (1995) tumour necrosis factor production in cats in response to lipopolysaccharide: an in vivo and in vitro study. Veterinary Immunology & Immunopathology. 49: 183-188
Shear MJ, Turner, FC. (1943) Chemical treatment of tumours; isolation of hemorrhagic-producing fraction from Serratia marcescens (Bacillus prodigious) culture filtrate. Journal of the National Cancer Institute. 4:81-87
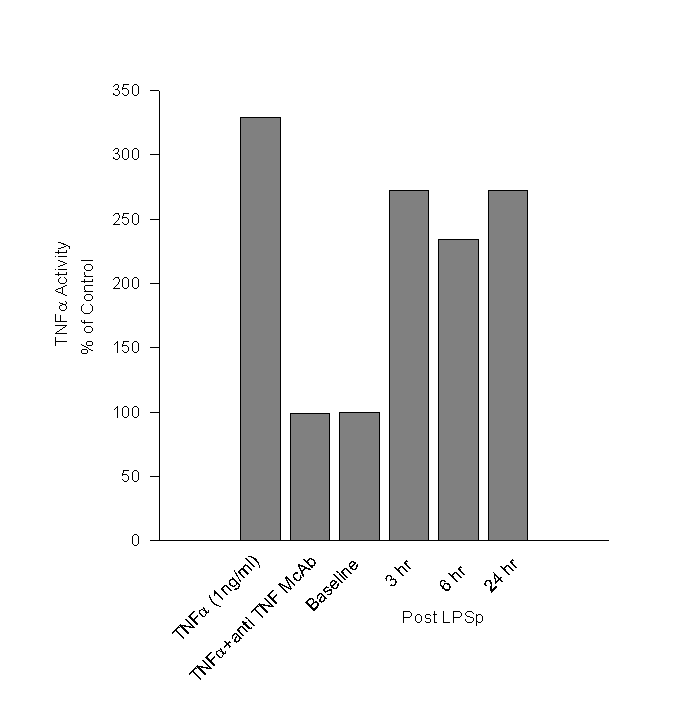
Figure
1. Cytotoxic Effect of TNFa
in Cat Serum
WEHI 164 clone 13 fibrosarcoma cells were treated with serum from the cat, collected pre- and 3, 6, and 24 hr post LPSp treatment. The assay was standardized using rhTNFa (1ng/ml) and shown to be specific with the use of a neutralizing anti-TNFa antibody. The results are expressed as a percentage of the activity detected in the baseline sample.
Haematological Findings
|
|
Baseline |
Pre-LPSp |
3 hrs Post LPSp |
6 hrs Post LPSp |
24 hrs Post LPSp |
|||||
|
WBC x 103/mm3 |
7.3 |
4.3 |
2.4 |
5.1 |
5.7 |
|||||
|
RBC x 103/mm3 |
10.9 |
7.3 |
6.2 |
6.2 |
10.0 |
|||||
|
Hgb (g/dl) |
12.9 |
9.9 |
9.5 |
9.7 |
8.6 |
|||||
|
HCT (%) |
35 |
29 |
29 |
29 |
26 |
|||||
|
WBC Differential (%) |
||||||||||
|
Segs |
41 |
50 |
25 |
69 |
49 |
|||||
|
Bands |
0 |
2 |
1 |
13 |
3 |
|||||
|
Lymphs |
53 |
41 |
67 |
14 |
42 |
|||||
|
Monos |
4 |
5 |
4 |
0 |
3 |
|||||
|
Eosins |
2 |
2 |
3 |
4 |
3 |
Body Weight and Temperature During Treatment
|
Day 0 |
Day 49 |
Day 98 |
Day 173 |
Day 214 |
||||||
|
Weight (kg) |
5.1 |
4.6 |
4.2 |
3.6 |
4.4 |
|||||
|
Temperature (oF) |
101.2 |
99.6 |
100.2 |
100.6 |
100.2 |
All pages copyright ŠPriory Lodge Education Ltd 1994-2004.
First published - November 15th 2000