V Boereª*, GR Paludob, T Piantaª, G Canaleª, C Tomazª
ª Primate
Center and Department of Physiological Sciences, Institute of Biology, University
of Brasília, DF, 70910-900, Brazil.
b Veterinary Hospital, Department of Agronomy and Veterinary, University of
Brasilia, DF, 70910-900, Brazil.
*Corresponding
author: Tel.:55 061 3072294; fax: 55 061 2741251.
E-mail address: vanner@unb.br (V. Boere).
Correspondence should be sent to: (Department of Physiological Sciences, Institute
of Biology, University of Brasilia, DF, 70910-900, Brazil; vanner@unb.br)
This study investigated the haematological state of captive marmosets submitted
to a chronic psychogenic stress, an environmental enrichment, or a control condition.
Blood samples were collected, one prior to and one after treatments, and were
analyzed for the total erythrocyte (ERY x 106/ l) and leucocyte count (LEU x
103/ l). Peripheral blood smears were used to determine LEU cells differentiation.
For all three groups, during the post-treatment phase, a significant increase
in monocyte (MONO) count was observed, while lymphocyte (LYM) concentration
decreased. Additionally, a higher MONO count was verified in the control group,
compared to the stressed marmosets, suggesting a possible higher mobilization
of the marginal immune compartment. Glucocorticoid resistance, common in marmosets,
may have provided these primates with a physiological defense against the effects
of chronic psychogenic stress. Finally, the enrichment procedures adopted failed
to significantly enhance the marmosets' immune response. Specific conditions
in which the animals were maintained are thought to have diminished attempts
to improve the quality of their housing conditions.
Keywords: blood cells, immune response, callithrichidae.
Stress, an adaptive mechanism of living organisms, is a highly variable phenomenon
(Sapolsky, 1993). Captivity for wild animals characterizes a kind of source
of stress due, for instance, to space restrictions, reduced social interactions,
and decreased specie-specific behaviors. As a result, significant changes may
be observed, including severe physiological disturbances, self-isolation, depression,
stereotyped behaviors, and death (Sapolsky, 1993; Boere, 2001). Additionally,
various pathologies are known to occur, such as diabetes, consumptive disorders,
osteoporosis, arteriosclerosis, gastric-duodenal ulcers (Sapolsky, 1993; Johnson
et al., 1996). Diminished immune response is one of the most frequently observed
consequence of prolonged or intense stress (Leonard & Song, 1996; Sternberg
& Gold, 1997; Schapiro et al., 2000).
In order to reduce these stress effects, spatial and social improvements should
be implemented and designed to satisfy an animal's physical and behavioral requirements.
Environmental enrichment (Boere, 2001), has been shown to improve various physiological
parameters of primates, including weight gain, decreased need of surgical interventions,
and increased reproductive turnover (reviewed in Poole, 1991; Boere, 2001),
as well as immune response (Coe & Ershler, 2001). Periodical variations
in housing conditions are thought to reduce the incidence of boredom due to
captivity, hence improving the animals' well-being (Boere, 2001). Enrichment
procedures have thus been increasingly implemented in species raised in captive
conditions, particularly non-human primates.
Stress-related responses in non-human primates, and their ensuing adaptive value,
can be evaluated by means of haematological indicators, namely leucocyte count
and traffic (Boccia Et Al., 1992; Dhabhar Et Al., 1995; Leonard & Song,
1996). Leucocytes are intensely mobilized during acute stress via adrenal catecholamines
and glucocorticoids. Chronic stress conditions(,) induce changes in leucocyte
dynamics, though their mechanisms are not fully established, and have been poorly
investigated in non-human primates (Schapiro Et Al., 2000; Coe & Ershler,
2001). The use of haematological indicators in vivo, nonetheless, enables a
more comprehensive approach to evaluate the impact of psychogenic stress.
The marmoset, a neotropical anthropoid of the genus Callithrix, has been frequently
employed in biomedicine and behavioral investigations (Smith et al., 2001).
Callitrichids are known to be resistant to cortisol, although they demonstrate
high basal levels of circulating glucocorticoids (Brandon et al., 1989). Indications
of a hypercortisolemic condition is also absent in these small primates. Traits
such as these are presumed to benefit species selection when susceptible to
high risks of predation and living in unstable environments (Ferrari, 1993;
Barros et al., 2002). Furthermore, a rapid habituation to changes in the environment
typically depicts these primates' stress-related response, such as when confronted
with a conspecific intruder (French & Inglett, 1991), in incidents of social
subordination (Abbott et al., 1998), and during blood sampling restraints (Johnson
et al., 1996). Glucocorticoid turnover is also more pronounced in marmosets,
compared to Old World primates (Bahr et al., 2000). In spite of these particularities,
marmosets' physiological response to psychogenic stress has remained poorly
explored.
Accordingly, this study was designed to investigate the haematological response
of captive-raised glucocorticoid resistant-to-glucocorticoid primate - the cerrado`s
marmoset (Callithrix penicillata) - to stress and enrichment conditions. For
this purpose, marmosets were submitted for nineteen consecutive days to either
potential environmental enrichment manipulations or social isolations associated
to 'aversive psychogenic' procedures.
Subjects and Maintenance
Twenty-four captive-raised marmosets (C. penicillata), seventeen adults and
seven juveniles, distributed into well established groups of heterosexual pairs
or groups of three, served as subjects. Animals were maintained in indoor/outdoor
enclosures (2 x 2 x 1.3 m) furnished with a suspended wood nest-box (0.3 x 0.3
x 0.6 m), feeding platform 1.5 m from the ground, two natural perches, tree
trunk, and natural dry leaves covering the floor. Groups had olfactory, audible
and visible contact with each other, although no physical contact was possible.
All marmosets were fed once daily, at 6:30/7:30 to 17:30 h, with a mixture of
various chopped fresh fruits and puppy dog chow, enriched with vitamins and
boiled eggs. Water was available ad libitum.
Maintenance and testing occurred at the Primate Center, University of Brasília,
Brazil, conforming to the regulations and with authorization of the Brazilian
Institute of the Environment and Renewable Natural Resources - IBAMA (registration
number 1/53/1999/000006-2). The study was approved by the Animals Ethics Committee
of the Institute of Biology, University of Brasília, Brazil, and handling
of marmosets was supervised by a veterinarian (V.B.).
Experimental Design
Haematological data were collected during two experimental phases: pre- and
post-treatments. During the pre-treatment phase, a blood sample was collected
for each subject to determine the basal haematological profile (see below).
Following blood sample collection procedures, marmosets were randomly assigned
to one of three experimental groups: an environmental enrichment, psychogenic
stress, or a control condition. Following treatments (post-treatment phase),
a second blood sample was collected for each subject (see below).
The enriched group (group E: two adult males, three adult females, one juvenile
male and one juvenile female) received daily permanent or intermittent housing
improvements deemed essential for the environmental enrichment of captive primates
(Crepeau & Newman, 1991; Poole, 1991; Boere, 2001). Table 1 describes the
enrichment treatments and their order of presentation.
Table One: Description of the environmental enrichment procedures and order
of presentation.
| Day* | Enrichment | Description |
| 1 | Replacement | A wooden platform for sun bathing and 2 perches were placed at a medium height |
| 2 | Polyvinyl chloride tube | Tube (0.3m x 0.10m) was hung from the cage's wire mesh ceiling. |
| 3 | Rope | Rope was hung from the cage's wire-mesh ceiling |
| 4 and 11 | Food variations | Transparent plastic food container, containing small pieces of fresh fruit (strawberry and peach) unknown to the subjects or pieces of gelatin, was attached outside the cage's front wire mesh. |
| 5 | Larvae 1 | Transparent plastic food container, containing Tenebrio mollitor larvae was attached outside the cage's front wire mesh. |
| 6 | Trapeze | Wood perch (0.3m x 0.03m) was hung from the cage's wire mesh ceiling. |
| 7 and 14 | Food bin | Parakeet and parrot food bin (Trill, Waltham Effem, Brazil) were hung from the cage's wire mesh ceiling. |
| 8 and 20 | Exploratory apparatus | Open transparent plastic bottle (600 ml) was placed upon the cage's floor, containing Tenebrio mollitor larvae |
| 9 and 17 | Cage rearrangement | The 2 perches and the polyvinyl chloride tube were placed at a greater height |
| 10 and 16 | Fishing | Transparent plastic bottle (600 ml) with a small window opening and containing water and alevines, was attacehd outside the cage's front wire mesh |
| 12 | Cardboard tubes | 4 cardboard tubes (0.3m x 0.05m) placed upon the cage's floor |
| 13 | Larvae 2 | Transparent plastic food container, containing Tenebrio mollitor larvae covered with dry natural leaves, was attacehd outside the cage's wire mesh ceiling. |
| 15 | Sugar cane | peeled sugar cane stick was hung from the cage's wire mesh ceiling |
| 18 | Bird's nest | Commercial bird's nest was place dinside the cage at height, containing 3 hard boiled quail's eggs |
| 19 | Gum tree | Hollow piece of bamboo, containing honey, placed vertcially in the cage. |
| * Order of presentation for each day of the enrichment procedures |
Marmosets submitted to the psychogenic stress condition (group S: four adult
males, four adult females, one juvenile male and juvenile female) were individually
housed in cages (1 x 0.5 x 0.7 m) located in a different building than the original
colony room, containing a wood nest-box (0.15 x 0.15 x 0.3 m), two parallel
wood perches (0.015 x 0.7 m), food and water containers. Cages were covered
with an opaque plastic curtain to eliminate visual contact among subjects and
with the surrounding environment, however, still allowing proper light and ventilation
conditions. Each subject was submitted to a sequence of distinct daily stressor
presentations, as psychogenic stress is induced essentially by novelty, lack
of control and unpredictability (Sapolsky, 1993). Accordingly, order of presentation,
type of stressor and time of day were randomly assigned, occurring within the
marmosets' activity period (07:00-18:00 h), except for the 'involuntary awakening'
(Table 2). Stressor presentations consisted of raising the curtain that surrounded
the cage and exposing the subject to a pre-established stressor for sixty seconds.
Sixty seconds interval is thought to be sufficient to induce a state of prolonged
stress (Bercovitch et al., 1995). Following the sixty seconds exposure, the
curtain was lowered and this same procedure held with other marmosets of the
stressed group. Table 2 describes the stressors stimuli employed, some of which
were adapted from previous investigations (Clarke, 1991; Crepeau & Newman,
1991; French & Inglett, 1991; Carey et al., 1992; Blanchard et al., 1998),
while others were based on observations of an earlier pilot study held in our
colony with a different group of marmosets.
Table Two: Description of psychological stress procedures and odrer of presentation
| Day* | Stress | Description |
| 1 | Redistribution | Subjects were placed individually in cages with small nest box, perches and opaque plastic curtain around the cage |
| 2 and 14 | Gloves | Exposure to a pair of leather gloves normally used for restraint about 0.2 m from the front of the cage. |
| 3 | Lab coat | Exposure to white lab coat on back of a chair about 0.2 m from the front of the cage |
| 4 and 16 | Unfamiliar Observer | Unfamiliar human observer stood before the cage, staring at the subject |
| 5 | Involuntary awakening | Subject awakened during nightly rest at 22.00 h by turning on lights for 10 minutes |
| 6 | Black curtain | Black curtain placed over subject's cage |
| 7 and 17 | Net | Exposure to net usually used for capturing marmosets about 0.2 m from the front of the cage. |
| 8 | Alarm clock | Exposure to alarm clock about 0.2 m from the front of the cage until the end of the alarm sound |
| 9 and 18 | Dragging | Cage was dragged to another place and subsequently moved back to original position |
| 10 | Striking | Striking of the window and door of the isolation room |
| 11 | Light spot | Light spot from lantern shone through opaque plastic curtain surrounding cage |
| 12 and 20 | Fire | Exposure to metal tray with 10 lighted candles on bench 0.5 m from the front of the cage. |
| 13 | Observer in lab coat | Exposure to unfamiliar human observer wearing a human lab coat |
| 15 | Light flash | Intermittent flashing red light from a bicycle at 0.2 m from cage |
| 19 | Sham feeding | Food container placed at usual hour without any food in |
| * Order of presentation for each day of the stress procedures |
The control group (group C: three adult males, two adult female, two juvenile
males) was exposed to a neutral stimulus (a brick discretely placed in one corner
of the home cages' entrances) each day of the experiment. The neutral stimulus
remained in the home cage throughout the day. It was rapidly substituted by
a familiar observer every morning with one of two bricks allocated for each
control group.
Blood Sample Collection and Analysis
Each subject was captured in a net, injected with ketamine hydrochloride for
immobilization (10 mg/kg, intramuscularly; Vetaset, Baton Rouge, USA), and then
placed into a small transportation cage for 1-3 min, until effective anesthesia.
Subsequently, the marmoset was placed on a restraining apparatus, situated in
a room adjacent to the colony, while 0.3 ml-0.8 ml of blood was collected from
femoral vein into a disposable syringe. The sample was then rapidly transferred
to an ethylenediaminetetra-acetic acid (EDTA) anticoagulant tube, and analyzed
2-4 h later. Blood was collected between 7:00-10:00 h.
Total cell count of (ERY), (LEU) and leucocyte subpopulations (i.e. lymphocytes
(LYM), monocytes (MONO), eosinophils (EOS), and segmented neutrophils (SEG)
were evaluated manually using Wright-Giemsa stain. Cell counts were held in
an automated electronic veterinarian blood cell counter (CC 550, Celm, São
Paulo). Counting was performed by a experienced veterinarian at clinical laboratory
diagnosis (G. R. P.).
Statistical analysis
The marmosets' haematological profile of the three experimental conditions were
compared using the Kruskal-Wallis test for unrelated samples, followed by the
Mann-Whitney test whenever significant values were obtained. Within each group,
comparisons between the two experimental phases were held employing the Wilcoxon
test. Differences between capture intervals were analyzed by Student's t-test.
Analyses were based on two-tailed results, represented by mean standard error
and the level of significance was set at P 0.05.
Two female marmosets (one adult and one juvenile) died during psychological
stress manipulations (data no showed). The results are the analysis of twenty
and two marmosets resting. The captures procedures, prior to venepunctures,
lasted 41.28 ± 28.28 seconds for the first sample, and 58.5 ±
40.9 seconds for the second. Capture intervals did not vary significantly between
the first and second venepuncture (P = 0.091).
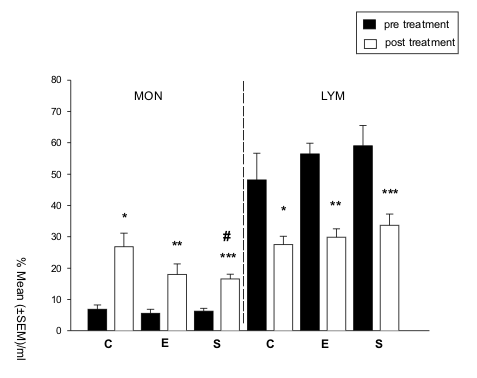
Comparisons among the groups E, S and C, prior to treatments, indicated similar
erythrocytes ERY (P 0.56), total leucocytes LEU (P 0.32), lymphocytes LYM (P
0.61), monocytes MONO (P 0.92), segmented neutrophils SEG (P 0.46) and eosinophils
EOS (P 0.39) concentrations among them.
Following tre
atments, however, MONO concentrations
were shown to significantly differ between groups ( 2 =6.093, d.f. = 2, P 0.05).
Post-hoc analysis showed that group S presented less MONO, when compared to
group C (nC = 6, nS = 9, Z = -2.659, P 0.008). Such difference was not observed
between group C and group E (nC = 6, nE = 7, Z = -1.502, P 0.13), neither between
group S and group E (nE = 7, nS = 9, Z = -0.106, P 0.91) (Fig. 1). Concentrations
of the remaining haematological parameters after treatments were not shown to
significantly differ from those observed during the pre-treatment phase (ERY:
P 0.79, total LEU: P 0.41, LYM: P 0.51, and SEG: P 0.86, EOS: P 0.51).
Analysis of the haematological profile after treatments for each group, revealed
that subject's MONO concentrations increased significantly, compared to the
pre-treatment phase (group C: Z = -2.023, P 0.04; group E: Z = -2.201, P 0.03;
group S: Z = -2.666, P 0.01; Fig. 1). Conversely, a significant decrease in
LYM concentration was observed following treatments in all three groups (group
C: Z = -2.023, P 0.05; group E: Z = -2.366, P 0.02; group S: Z = -2.666, P 0.01;
Fig. 1).
 |
Figure 1. Monocyte (MONO) and lymphocyte (LYM) concentration, analyzed as the percentage mean of cells/ml ( SEM), in the control (C), enriched (E) and stressed (S) groups, during the pre- and post-treatment phases. MONO: * P 0.04, ** P 0.03, *** P 0.01, # P 0.008; LYM: * P 0.043, ** P 0.02, *** P 0.01 (two-tailed Wilcoxon test). |
The erythrocytes (group C: P 0.68; group E: P 0.73; group S: P 0.76), total
LEU (group C: P 0.08; group E: P 0.12; group S: P 0.21), and EOS concentrations
(group C: P 0.31; group E: P 0.56; group S: P 0.15) were not found to significantly
differ in the three groups after treatments. SEG was not significantly different
in the group C and S (group C: P 0.68; group S: P 0.10), but in the group E
there was a tendency to increase after treatment (P 0.06).
The three experimental conditions (i.e. stress, environmental enrichment, control)
were found to alter the cerrado`s marmosets' (C. penicillata) haematological
profile. The effects observed following treatments were, in fact, associated
to distinct blood cell types that constitute the cell-mediated immune response.
Accordingly, a significant decrease in LYM content, while an increase in MONO
concentration was observed in the peripheral blood of all three groups after
treatments. Similarly, several studies have been reported that stressed animals
demonstrate a reduction in LYM number and proliferation of MONO (Leonard &
Song, 1996; Connor et al., 1997).
Nonetheless, marmosets' immune response to the experimental manipulations differed
between LYM and MONO. The decrease in LYM availability following treatments
did not differ between groups. On the other hand, a smaller MONO mobilization
was observed in the peripheral blood of the stress group, compared to the control
group. Consequently, MONO redistribution in our subjects may be due to the nature
of the experimental manipulation. Isolation and constant exposure to unpredictable
novel events (i.e. stress condition) seem to have had a greater impact on the
haematological response of cerrado's marmosets. Additionally, MONO are known
to be distributed in the blood and other immune compartments (i.e. marginal)
in humans. Dhabhar et al. (1995) suggested a marginal compartmentalization in
rats, reporting that physical restraint stress in rats, modified the number
and percentage of LYM and SEG in the peripheral blood, returning to normal basal
levels following a 3 h interval. The existence of a MONO marginal cellular compartment,
however, has not been reported in other Neotropical primate species, although
glucocorticoid administration was shown to induce monocytosis in dogs (Jain.,
2000). Accordingly, a marginal compartment is suggested to also be present in
nonhuman primates as marmosets.
Contrarily, total LEU, EOS, and SEG concentration were found not to differ between
the pre- and post-treatment phases, nor among groups. In spite of this, we do
not have a reasonable explanation for the tendency to increment of SEG in group
E after treatment. We can speculate a tendency to higher phagocytosis activity
of neutrophils in group E then group C and S, maybe in consequence to high blood
lost in the venepuncture. In fact, sometimes subjects of group E presented local
haematomes after venepuncture.
The erythrocytes concentration was also not altered by any of the experimental
manipulations. A change in MONO proportion was observed, however, probably due
to the action of adrenal glucocorticoids. These, in turn, influence mitogenesis,
chemical communication and adhesiveness of blood cells involved in the immune
response (Leonard & Song, 1996; Turnbull & Rivier, 1999; Bauer et al.,
2001). Furthermore, activation of the autonomic nervous system, with a consequent
acute release of catecholamines into the peripheral blood, modulates the LEU
response via receptors located on blood vessels and lymphatic organs (Bauer
et al., 2001). Although many factors are known to alter the immune response,
the nature of the stressor seems to influence the level and type of LEU mobilization.
For instance, psychogenic stress alters the distribution of immune cell sub-types
in humans (Uchakin et al., 2001). An increase in LYM cytolysis has also been
shown in rhesus monkeys submitted to psychogenic stress (i.e. social isolation),
as opposed to physical stressors (i.e. laparoscopy) (Lemieux et al., 1996).
Importantly, in spite of the decrease in LYM, its concentrations, regardless
of treatment, remained above the minimum physiological level of 3 x 109/L reported
for common marmosets (Hawkey et al., 1982). Therefore, LYM decrease was not
sufficient to induce pathologic lymphopaenia, even in subjects submitted to
severe stress. Glucocorticoid resistance, a physiological condition of high
basal plasmatic cortisol, without pathological signs (Brandon et al., 1989)
commonly observed in genus Callithrix (Johnson et al., 1996), may have influenced
this result. Mitosis of cells precursors of LYM and MONO couldn't be inhibited
because the receptor hormone attachment is weak in glucocorticoid resistance
primates (Scammel et al., 2001). However further studies are necessary to test
this assumption.
Enrichment procedures presently adopted failed to significantly enhance the
marmosets' immune response, compared to the control group. Specific conditions
in which the animals were maintained may have minimized improvements made during
this experimental manipulation. Marmosets employed in this study were distributed
in socially compatible groups and housed in enclosures furnished with substrates
deemed appropriate for an enriched environment for these simians. In addition,
the Primate Center is located within an forested protected area. Marmosets were
therefore exposed to the same conditions present in their natural habitat, thus
creating an added factor to their well-being.
Lastly, blood cell counts observed prior to treatments were similar to those
previously reported for cerrado`s marmosets (Diniz, 1997) and common marmosets
(C. jacchus) (Hawkey et al., 1982; Yarbrough et al., 1984).
Studies analyzing haematological modifications, incorporating diagnostic and
prognostic data, may be useful to increase reproductive rates, survival and
well-being of captive-raised animals. Further detailed investigations on the
dynamics of LEU and its sub-types, as well as on antibodies and cytokine production,
are deemed necessary. This is especially true since, compared to studies on
the effects of environmental enrichment procedures, biobehavioral investigations
analyzing the effects of psychogenic stress in neotropical primates are presently
scarce.
In short, we conclude that the psychological stress diminishes the immune response
of LYM and MONO in cerrado's marmoset, but high frequency of innovations in
a environmental enrichment program seems not to improve the immune response.
Despite the severe stressors, putative glucocorticoid-resistance may confer
an adaptive immune advantage of marmosets towards the frequent and unpredictable
challenges present in captivity.
We are grateful to Mr. R. Oliveira and Mr. W. Vargas for the care of animals.
We thank to Dr I. O. Silva and Dr M. Barros to helpful comments on this paper.
The investigation was supported CAPES/PICDT fellowship to V.B., and PIBIC/DPP/CNPq
fellowship to G. C. and T. P. FINATEC/DF was a partial financial supporter.
Abbott DH, Saltzman W, Schultz-Darken NJ, Tannenbaum PL. (1998).
Adaptations to subordinate status in female marmoset monkeys. Comparative Biochemical
Physiology C Pharmacology and Toxicology .119, 261-274.
Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. (2000). Comparative
aspects of the metabolism and excretion of cortisol in three individual nonhuman
primates. General Comparative Endocrinology. 117, 427-438.
Barros M, Boere V, Mello Jr EL, Tomaz C. (2002). Reactions to potential predators
in captive-born marmosets (Callithrix penicillata). International Journal Primatology.
23, 443-454.
Bauer ME, Perks P, Lightman SL, Shanks N. (2001). Restraint stress is associated
with changes in glucocorticoid immunoregulation. Physiology and Behavior. 73,
525-532.
Bercovitch FB, Hauser MD, Jones JH. (1995). The endocrine stress and alarm vocalizations
in rhesus macaques. Animal Behavior. 49, 1703-1706.
Blanchard RJ, Hebert M, Sakai RR, McKittrick C, Henrie A, Yudko E, McEwen B,
Blanchard DC. (1998). Chronic social stress: changes in behavioral and physiological
indices of emotion. Aggressive Behaviour. 24, 307-321.
Boccia ML, Laudenslager ML, Broussard CL, Hijazi AS. (1992). Immune responses
following competitive water tests in two species of macaques. Brain Behavior
and Immunity. 6, 201-213.
Boere, V. (2001). Primates: behavior and environmental enrichment. In: Fowler
ME, Cubas ZS, editors. Biology, Medicine, and Surgery of South American Wild
Animals. Ames: Iowa University Press. p. 263-267.
Brandon DD, Markwick AJ, Chrousos GP, Loriaux DL. (1989). Glucocorticoid resistance
in humans and nonhumans primates. Cancer Research. 49, 2203-2211.
Carey GJ, Costall B, Domeney AM, Jones DNC, Naylor RJ. (1992). Behavioural effects
of anxiogenic agents in the common marmoset. Pharmacology Biochemistry and Behavior.
42, 143-153.
Clarke, AS. (1991). ACTH and glucocorticoid responses under two conditions of
stress in macaques. American Journal of Primatology. 25, 115-124.
Coe CL, Ershler WB. (2001). Intrinsic and environmental influences on immune
senescence in the aged monkey. Physiology and Behavior. 73, 379-384.
Connor TJ, Kelly JP, Leonard BE. (1997). Forced swim test-induced neurochemical
endocrine and immune changes in the rat. Pharmacology Biochemistry and Behavior.
58, 961-967.
Crepeau LJ, Newman JD. (1991). Gender differences in reactivity of adult squirrel
monkeys to short-term environmental challenges. Neurosciences Biobehavioral
Review. 15, 469-471.
Dhabhar FS, Miller AH, McEwen BS, Spencer RL. (1995). Effects of stress on immune
cell distribution dynamics and hormonal mechanisms. Journal of Immunology. 154,
5511-5527.
Diniz LSM. (1997). Primatas em cativeiro, manejo e problemas veterinários
(Primates in captivity, management and veterinary problems. São Paulo:
Ícone. 196 pp.
Ferrari SF. (1993). Ecological differentiation in the Callitrichidae In: Marmosets
and Tamarins: Systematics Behaviour and Ecology. Rylands AB, editor. Oxford:
Oxford University Press. 314-328.
French J, Inglett BJ. (1991). Responses to novel social stimuli in callitrichid
monkeys: a comparative perspective In: Primate Responses to Environmental Change.
Box H, editor. London: Chapman & Hall. 275-294.
Hawkey CM, Hart MG, Jones DM. (1982). Clinical haematology of the common marmoset
Callithrix jacchus. American Journal of Primatology. 3, 179-199.
Jain, NC. (1986). Schalm's Veterinary haematology. 4th ed. Lippincott: Williams
& Wilkins. 1221 pp.
Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. (1996).
The biobehavioral consequences of psychogenic stress in a small social primate
(Callithrix jacchus jacchus). Biological Psychiatry. 40, 317-337.
Lemieux AM, Coe CL, Ershler WB, Karaszewski JW. (1996). Surgical and psychological
stressors differentially affect cytolytic responses in the rhesus monkey. Brain
Behavior and Immunity. 10, 27-43.
Leonard BE, Song C. (1996). Stress and the immune system in the etiology of
anxiety and depression. Pharmacology, Biochemistry and Behavior. 54, 299-303.
Poole T. (1991). Criteria for the provision of captive environments In: Primate
responses to environmental change. Box H, editor. London: Chapman Hall. 357-74.
Sapolsky RM. (1993). Neuroendocrinology of the stress-response In: Behavioral
Endocrinology. Becker JB, Breedlove SM, Crews D, editors. Cambridge: The MIT
Press. 287-324
Scammel JG, Denny WB, Valentine DL, Smith DF. (2001). Overexpression of the
FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance
in three new world primates. General and Comparative Endocrinology. 124, 152-165.
Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. (2000). A comparison of cell-mediated
immune responses in rhesus macaques housed singly in pairs or in groups. Applied
Animal Behavior Science. 68, 67-84.
Smith D, Trennery P, Farningham D, Klapwijk J. (2001). The selection of marmoset
monkeys .Callithrix jacchus. in pharmaceutical toxicology. Laboratory Animals.
35, 117-130.
Sternberg EM, Gold PW. (1997). The mind-body interaction in disease. Scientific
American, Special Issue: 8-15.
Turnbull AV, Rivier CL. (1999). Regulation of the hypothalamic-pituitary-adrenal
axis by cytokines: actions and mechanisms of action. Physiology Reviews. 79,
2-71.
Uchakin PN, Tobin B, Cubbage M, Marshall G, Sams C. (2001). Immune responsiveness
following academic stress in first-year medical students. Journal of Interferon
and Cytokine Research. 21, 687-694.
Yarbrough LW, Tollett JL, Montrey RD, Beattie RJ. (1984). Serum biochemical
haematological and body measurement data for common marmosets (Callithrix jacchus
jacchus). Laboratory Animal Science. 34, 276-280.
All pages copyright ©Priory Lodge Education Ltd 1994-2004.
First Published September
2003