Double blind randomized controlled trial on the effects of mannitol on the postreperfusion syndrome during orthotopic liver transplantations.
Authors:
Greg Dembo, MD1
Lin Chen, MS2
Youri Vater, MD, PhD1
Norman E. Breslow, PhD2
Kenneth Martay, MD1
ABSTRACT
Objective. The postreperfusion syndrome during orthotopic liver transplantations is a severe decrease in blood pressure occurring within minutes after reperfusion of the liver graft. It can cause early graft dysfunction and multi-organ failure in the recipient. Evidence suggests that the generation of oxygen free radicals in the liver graft during its ischemic time, which are then flushed into the circulation of the recipient after reperfusion of the liver graft, causes the profound hypotension of the postreperfusion syndrome. Based on this assumption, mannitol - an oxygen free radical scavenger and osmotic diuretic - was investigated for attenuating the postreperfusion syndrome.
Materials and Methods. Double blind randomized controlled trial enrolling 50 patients undergoing orthotopic liver transplantations. Half of the patients received mannitol and half of them placebo (0.9% NACL). Decrease in mean arterial blood pressure as well as total amounts of epinephrine and phenylephrine at reperfusion of the liver graft and for 60 minutes post reperfusion were recorded. Linear regressions and t-tests were used for statistical analysis.
Results. The postreperfusion syndrome was defined as a decrease in the mean arterial blood pressure of >30% below the baseline value in the recipient occurring within 5 minutes after reperfusion of the liver graft and lasting for 1-60 minutes. The mannitol group required significantly less vasopressor support than the placebo (0.9% NACL) group to maintain a mean arterial blood pressure of ³ 70 mmHg following reperfusion of the liver graft.
Conclusion. Mannitol attenuated the postreperfusion syndrome during orthotopic liver transplantations.
INTRODUCTION
The postreperfusion syndrome (PRS) during orthotopic liver transplantations (OLT) has been defined as a decrease in the mean arterial blood pressure of >30% below the baseline value in the recipient occurring within minutes after reperfusion of the liver graft.(1) The PRS is one of the major problems affecting the outcome of OLT. Shortly after the reperfusion of the liver graft, a reduction in the mean arterial blood pressure (MABP) and in the systemic vascular resistance, as well as an increase in the cardiac index can be observed.(2) The PRS can cause early graft dysfunction and may culminate in multi-organ failure in the recipient.(2,3) In other patients, the cardiac index is decreased either due to a reduced myocardial contractility,(4) or due to a lower heart rate.(5,6) Profound hypotension is another event,(2,7) of which the exact mechanisms are still not fully understood.(8) One of the explanations for the hemodynamic changes focuses on the actions of oxygen free radicals (OFR) generated in the liver graft during its ischemic time,(8-11) which are released into the recipientÕs circulation at reperfusion of the liver graft.(12-15) OFR are chemically unstable compounds which exert their toxic effects by reacting with lipids, proteins, and nucleotides to produce oxidized (unstable) compounds.(16) The abrupt oxygen influx into the ischemic hepatic tissue upon reperfusion together with the leakage of xanthine oxidase and its purine substrates can generate superoxide anions (O2ø), H2O2 and áOH, of which the latter is the most toxic one.(9) Additionally there may be a secondary production of áOH radicals in the circulation, all of which could explain remote organ injuries.(17) Animal studies demonstrated that OFR induce vasodilation in the aortic rings in rabbits,(18) and loss of normal vascular tone in rats.(19) We report the results of a double blind randomized controlled trial comparing the effects of mannitol versus placebo (0.9% NACL) on the PRS during OLT.
MATERIALS AND METHODS
The study was designed to investigate whether mannitol will attenuate the occurrence of the PRS during OLTs in comparison to placebo (0.9% NACL). After approval by the local human subjects committee, written informed consent of 50 patients scheduled for OLT was obtained. Immediately before transplantation the patients were randomized by the hospital pharmacy through a computer generated random number table into two groups – mannitol and placebo (0.9% NACL). The solutions produced by the hospital pharmacy, 2 bags for each patient, were only labeled as Ôstudy fluidÕ and contained either mannitol 20% or normal saline according to the calculated dry bodyweight of the patient. (Mannitol 20%: 1g = 5ml solution. For a 70kg patient, two bags of 70x5=350ml study fluid (mannitol or placebo) would be prepared, altogether 700ml).
All patients were prepared according to the OLT protocol in use at the University of Washington Medical Center, Seattle, Washington, USA. All patients received midazolam (1-2mg, iv) and after connection of the patient to a fully computerized monitoring and record keeping system consisting of 5-lead ECG, non-invasive blood pressure, and pulseoximeter (Datex-Ohmeda¨, Madison, Wisconsin, USA), anesthesia was induced with fentanyl (250μg), propofol (2mg/kg-1), and cisatracurium (0.2mg/kg-1). After endotracheal intubation was confirmed, anesthesia was maintained with isoflurane in oxygen, and continuous infusions of fentanyl (2-5μg/kg-1/min-1) and cisatracurium (1μg/kg-1/min-1). No nitrous oxide was used during surgery. One or two radial artery lines were inserted for continuous invasive arterial blood pressure monitoring, and a MACª Two Lumen Central Venous Access line (Arrow International Inc.ª, Reading, Pennsylvania, USA) was placed in the right internal jugular vein for fluid delivery. Through the third port in that line a Swan-Ganz CCOmbo CLO/SVO2/VIP thermodilution catheter (Edwards Lifesciences LLC, Irvine, California, USA) was floated for continuous central venous pressure and pulmonary artery pressure monitoring. Antibiotics and immunosuppressants were given, and an aprotinin infusion (2,000,000 units loading dose over 1 hour, followed by 500,000 units per hour until end of surgery) and a dopamine infusion (3μg/kg-1/min-1 until end of surgery) were commenced. Vasopressor infusions (phenylephrine, epinephrine) were started if the MABP decreased < 70 mmHg for more than 1 minute. Blood products were infused according to clinical and laboratory parameters. All patients received albumin 5%, 500-1000 ml, during the anhepatic phase during which piggyback technique was employed in all cases. No veno-venous bypass was used during any of the orthotopic liver transplantations in this study. About 10 minutes prior to reperfusion of the liver graft, an arterial blood gas sample was taken to correct calcium levels and metabolic acidosis if required. At the same time, each patient was infused a bag of 5ml/kg pre-prepared study fluid as bolus. If the patient belonged to the mannitol group, he/she received mannitol 1g/kg (mannitol 20% solution: 1g=5ml); if the patient belonged to the placebo group, he/she received 0.9% NACL, 5 ml/kg. At the moment of reperfusion, in each patient a continuous infusion of a second bag of the same pre-prepared study fluid (mannitol 20% or 0.9% NACL) was started for the duration of 1 hour. If after reperfusion of the liver graft the MABP dropped < 70 mmHg, phenylephrine and epinephrine boluses were given and infusions of the same drugs were started until the MABP was ³ 70 mmHg. The amount of vasopressors given was recorded over the first hour after reperfusion of the liver graft. At the end of surgery the patients were extubated either in the OR or in ICU.
The statistical analysis for this study was performed by analyzing the difference between the mannitol and the placebo group in mean MABP levels over time by linear regression analysis containing a 3 degree of freedom spline term to represent the effects of time, a treatment indicator to represent the average difference between the mannitol and placebo groups, and a linear treatment by time interaction term to represent the treatment difference in the rate of change. Standard errors of the regression coefficients were adjusted for the repeated measurements made on each patient using the method of generalized estimating equations.(20) Ordinary t-tests and chi-squared tests for independence were used to compare treatment groups with respect to means and proportions based on a single measurement per patient.
RESULTS
All patients enrolled in the study were ASA 3. A t-test statistical analysis of the clinical and demographic data of both study groups is presented in table 1. The data of both groups are not significantly different from each other.
Table 2 depicts the intraoperative fluid balances of both study groups. Over the entire length of surgery the average non-study related fluid replacement in both groups was the same (p=0.96). The average blood loss in the mannitol group was about 250 ml higher than in the placebo group (p=0.16), but the average urine output in the mannitol group was more than double that of the placebo group (p=0.0). If the total intraoperative fluid balances between both groups are compared, the mannitol group has a significantly higher fluid loss (blood loss + urine output) than the placebo group (p= 0.0).
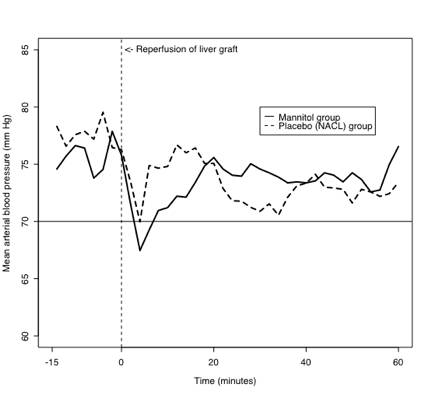
In this study the postreperfusion syndrome was defined as a decrease in the mean arterial blood pressure of >30% below the baseline value in the recipient occurring within 5 minutes after reperfusion of the liver graft and lasting for up to 60 minutes, which was the length of time up to which hypotension (MABP <70mmHg) could be observed after reperfusion of the liver graft. Earlier studies identified marked hypotension as the main symptom of the PRS. The average MABP of the mannitol and the placebo group ranging from 15 minutes prior to reperfusion of the liver graft to 60 minutes after reperfusion of the liver graft are depicted in figure 1. Since the study was aimed at the effects mannitol may have on the PRS during OLT, only the period from 0-60 minutes after reperfusion of the liver graft was statistically analyzed. Although the average MABP for the mannitol group dropped to a slightly lower level at 5 minutes following reperfusion than for the placebo group, and increased thereafter at a slightly faster rate, these apparent differences between treatment groups were not statistically significant (p=0.50 for joint test of treatment and treatment-time interaction).
The total amount of epinephrine and phenylephrine given in both the mannitol and the placebo group over the first 60 minutes of the postreperfusion period was also analyzed. Since epinephrine and phenylephrine are vasopressors used to prevent hypotension, different doses of these drugs used in both groups might indicate differences in efficacy of mannitol and placebo. Based on chi-square tests of independence, there were no significant differences in the proportions of patients in the mannitol and placebo group who received epinephrine (p=0.99) or phenylephrine (p=0.74) after reperfusion of the liver graft. Table 3 shows the results of two sample t-tests, assuming unequal variances, comparing the total amounts of epinephrine and phenylephrine given to both groups during the postreperfusion period. The mean epinephrine and phenylephrine doses delivered to the placebo group were higher than those delivered to the mannitol group. The average dose of phenylephrine delivered to the 14 placebo patients who required such intervention was significantly higher (> 1000 μg) than that delivered to the 12 mannitol treated patients who needed it (p=0.03). The mean length of delivery time in the placebo group was also longer than in the mannitol group, but that difference was not statistically significant.
DISCUSSION
Various treatments have been recommended, individually or in combination, for the postreperfusion syndrome which is a major cause of morbidity and mortality in orthotopic liver transplantations, and can result in multi-organ failure.(21)
Atropine was suggested for the prevention of bradycardia induced hemodynamic changes during liver graft reperfusion, but prevented only bradycardia itself.(7) Prophylactic intravenous application of potassium, calcium, and sodium bicarbonate reduced the duration but not the occurrence of myocardial depression associated with the postreperfusion syndrome.(22) It is currently believed that vasodilation plays an important role in the occurrence of the PRS since vasopressors were shown to successfully counteract the severe decrease in mean arterial blood pressure after liver graft reperfusion.(23-25) Vasopressors, however, also reduce the splanchnic venous blood flow to the liver graft and can thus jeopardize the long-term survival of the graft. The severe vasodilatation during the reperfusion phase is attributed to the activity of OFR in the circulation which are generated within the liver graft during its ischemic time.(8-11) Several recent studies in animals as well as in isolated perfused organs demonstrated that OFR cause vessels to lose their physiological reactivity to physical factors and to humoral effects. OFR scavengers were shown to effectively attenuate, or even prevent, the effects of the postreperfusion syndrome during liver transplantations on other organs.(21,26) Following promising results in animals studies, clinical studies successfully used OFR scavengers such as n-acetylcysteine and methylene blue during the postreperfusion phase in orthotopic liver transplantations in humans.(8,27)
Mannitol possesses, besides its osmotic diuretic properties, also hydroxyl radical scavenging properties that have been demonstrated in animal studies.(28) Mannitol inhibits the spontaneous aggregation of human platelets when subjected in vitro to conditions of anoxia and reoxygenation,(29) and reduces the production of H2O2 in patients undergoing coronary artery bypass grafting.(30) It was also demonstrated that mannitol provides neuroprotection by preventing both necrosis and apoptosis of cells after transient cerebral ischemia,(31) that it protects ischemic muscles from reperfusion injury,(32) and that it prevents liver-reperfusion-induced lung injury.(9,21) In other animal studies, mannitol attenuated the negative effects of hydroxyl radicals on cerebral arterioles in cats,(33) and completely restored the tonus of aortic rings affected by áOH in rabbits and rats.(18,19)
The main blood loss during orthotopic liver transplants usually occurs during the resection phase of the recipientÕs liver and/or during reperfusion of the liver graft. Blood loss and volume replacement can influence the reperfusion syndrome so that in patients with higher total volume losses one would expect increased cardiovascular instability. Despite the higher total fluid loss in the mannitol group, the mannitol group required less vasopressors than the placebo group to maintain a MABP ³ 70 mmHg. There was no statistically significant difference in the mean blood pressure measurements over time after reperfusion between the mannitol and placebo group (p > 0.1).
There were also no differences in the proportions of patients who received epinephrine or phenylephrine, respectively. However, the average amount of phenylephrine delivered to patients who needed the drug to increase their BP was significantly lower in the treatment than in the placebo group (p = 0.031). The amount of epinephrine and the time of delivery were lower (respectively, shorter) too in the mannitol group than in the placebo group, but these results were not statistically significant. In vivo measurements of free radicals were not done for this study because that had required the preoperative oral application of acetylsalicylic acid, 1 g, to the patients.(34,35) This, however, would have been contraindicated in patients with already preoperatively abnormal blood clotting parameters and the idea was met by strong opposition by the surgeons as well. The MELD scores in both study groups were on average not very high. The reasons for that are twofold: the waiting time for liver transplantations in Seattle is shorter than the national average so that patients are healthier when undergoing OLT, and patients with high MELD scores are often encephalopathic, therefore unable to sign an informed consent and were thus excluded from the study.
In conclusion, the here presented study showed that mannitol attenuated the postreperfusion syndrome during OLTs. The mannitol regime described here has become a standard part of the OLT protocol in Seattle, but we encourage more studies into the effects of mannitol during OLTs to confirm the here presented results.
REFERENCES
1. Aggarwal S, Kang Y, Freeman J, DeWolf AM, Begliomini B. Is there a post-reperfusion syndrome? Transplant Proc 1989; 21: 3497-3499.
2. Aggrawal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Post-reperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc 1987;199(suppl): 54-55.
3. Aggrawal S, Kang Y, Freeman JA, Fortunato FL Jr, Pinsky MR. Post-reperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care 1993; 8: 154-160.
4. Webster NR, Bellamy MC, Lodge JP, Sadek SA. Haemodynamics of liver reperfusion: comparison of two anaesthetic techniques. Br J Anaesth 1994; 72: 418-421.
5. Estrin JA, Belani KG, Ascher NL, Lura D, Payne W, Najarian JS. Hemodynamic changes on clamping and unclamping of major vessels during liver transplantation. Transplant Proc 1989; 21: 3500-3505.
6. Brems JJ, Takiff H, McHutchinson J, Collins D, Biermann LA, Pockros P. Systemic versus nonsystemic reperfusion of the transplanted liver. Transplantation 1993; 55: 527-529.
7. Acosta F, Sansano T, Contreras RF, et al. Atropine prophylaxis of the postreperfusion syndrome in liver transplantation. Transplant Proc 1999; 31: 2377.
8. Koelzow H, Gedney JA, Baumann J, Snook NJ, Bellamy MC. The effect of methylene blue on the hemodynamic changes during ischemia reperfusion injury in orthotopic liver transplantation. Anesth Analg 2002; 94: 824-829.
9. Weinbroom AA, Hochhauser E, Rudick V, et al. Multiple organ dysfunction after remote circulatory arrest: common pathway of radical oxygen species? Journal of Trauma-Injury Infection and Critical Care 1999; 47: 691-698.
10. Castro GD, Delgado de Layno AM, Castro JA. Liver nuclear ethanol metabolizing systems (NEMS) producing acetaldehyde and 1-hydroxyethyl free radicals. Toxicology 1998; 129: 137-144.
11. Goode HF, Webster NR, Howdle PD, et al. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology 1994; 19: 354-359.
12. Shirasugi N, Wakabayashi G, Shimazu M, et al. Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury. Transplantation 1997; 64: 1398-1403.
13. Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury of the liver. Journal of Molecular Medicine 1999; 77: 577-596.
14. Serrachino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. American Journal of Surgery 2001; 181: 160-166.
15. Iwamoto A, Egashira T, Takayama F, Yamanaka Y, Noguchi T. Change in free radical-related substances in plasma following ischemia-reperfusion in rat liver. Pathophysiology 2002; 8: 167-174.
16. Vendemiale G, Grattagliano I, Altomare E. An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res 1999; 29: 49-55.
17. Chevion M, Jiang Y, Har-el R, Berenstein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci USA 1993; 90: 1102-1106.
18. Bharadwaj L, Prasad K. Mechanism of hydroxyl radical-induced modulation of vascular tone. Free Radic Biol Med 1997; 22: 381-390.
19. Weinbroum AA, Kluger Y, Rudick V. Impairment of aortal tone by no flow – reflow conditions and its partial amelioration by mannitol. Annals of Thoracic Surgery 200; 69: 1439-1444.
20. Liang KY, Zeger SL. Longitudinal data analysis using general linear
models. Biometrika 1986; 73: 13-22.
21. Weinbroum AA, Shapira I, Ben-Abraham R, Szold A. Mannitol dose-dependently attenuates lung reperfusion injury following liver ischemia reperfusion: a dose-response study in an isolated perfused double-organ model. Lung 2002; 180: 327-338.
22. Acosta F, Diaz J, Sansaro T, et al. Prophylactic treatment of metabolic alterations during revascularization in liver transplantation. Transplant Proc 1994; 26: 3667-3668.
23. Seifert RD, Kang YG, Begliomini B, Miller SR. Baseline cardiac index does not predict hemodynamic instability during orthotopic liver transplantation. Transplant Proc 1989; 21: 3523-3524.
24. Acosta F, Sansano T, Contreras RF, et al. Phenylephrine treatment of the postreperfusion syndrome in liver transplantation. Transplant Proc 1999; 31: 2373-2374.
25. Acosta F, Rodriguez MA, Sansano T, et al. Need for inotropic and/or vasopressor drugs during liver transplantation. Transplant Proc 1999; 31: 2403-2403.
26. Regoli F, Winston GW. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite, peroxyl radicals, and hydroxyl radicals. Toxicology and Applied Pharmacology 1999; 156: 96-105.
27. Weigand MA, Plachky J, Thies JC, et al. N-acetylcysteine attenuates the increase in alpha-glutathione S-transferase and circulating ICAM-1 and VCAM-1 after reperfusion in humans undergoing liver transplantation. Transplantation 2001; 72: 694-698.
28. Dunphy G, Richter HW, Azodi M, et al. The effects of mannitol, albumin, and cardioplegia encancers on 24-h rat heart preservation. Am J Physiol 1999; 276: H1591-H1598.
29. Leo R, Pratico D, Iuliano L, et al. Platelet activation by superoxide anion and hydroxyl radicals intrinsically generated by platelets that had undergone anoxia and then reoxygenated. Circulation 1997; 95: 885-891.
30. Yang MW, Lin CY, Hung HL, et al. Mannitol reduces plasma hydrogen peroxide free radical in patients undergoing coronary artery bypass graft surgery [Chinese]. Ma Zui Xue Za Zhi Anesthesiologica Sinica 1992; 30: 65-70.
31. Korenkov AI, Pahnke J, Frei K, et al. Treatment with nimodipine or mannitol reduces programmed cell death and infarct size following focal cerebral ischemia. Neurosurg Rev 2000; 23: 145-150.
32. Schlag MG, Clarke S, Carson MW, Harris KA, Potter RF. The effect of mannitol versus dimethyl thiourea at attenuating ischemia/reperfusion-induced injury to skeletal muscle. J Vasc Surg 1999; 29: 511-521.
33. Kontos HA, Hess ML. Oxygen radicals and vascular damage. Adv Exp
Med Biol 1983; 161: 365-375.
34. Coudray C, Talla M, Martin S, Fat™me M, Favier A. High-performance liquid
chromatography – electrochemical determination of salicylate hydroxylation products as an in vivo marker of oxidative stress. Anal Biochem 1995; 227: 101-111.
35. Coudray C, Favier A. Determination of salicylate hydroxylation products as
an in vivo oxidative stress marker. Free Radic Biol Med 2000; 29: 1064-1070.
Table 1. Mannitol and Placebo (0.9% NACL) study groups: clinical and demographic data
| Age (years) |
BMI (kg/m2) |
MELD score |
Liver graft ischemic time: COLD (h/min) |
Liver graft ischemic time: WARM (h/min) |
Liver graft ischemic time: TOTAL (h/min) |
Duration of surgery (h/min) |
||
| Mannitol group |
Range |
18-61 |
20-37 |
6-36 |
2h41min- 11h26min |
0h24min- 0h46min |
3h11min- 12h01min |
2h57min- 5h04min |
| Mean |
49.7 |
29.3 |
20.1 |
6h19min |
0h34min |
6h54min |
5h12min |
|
| SD |
9.04 |
4.72 |
7.50 |
2h09min |
0h06min |
2h08min |
1h03min |
|
| Placebo group |
Range |
25-69 |
20-38 |
12-32 |
2h28min- 14h16min |
0h18min-0h45min |
2h46min- 14h44min |
2h40min- 9h59min |
| Mean |
52 |
27.8 |
20 |
6h20min |
0h32min |
6h51min |
5h20min |
|
| SD |
9.37 |
4.57 |
4.80 |
2h48min |
0h08min |
2h49min |
1h36min |
|
| P-value |
0.38 |
0.26 |
0.96 |
0.98 |
0.23 |
0.94 |
0.73 |
|
Table 2. Intraoperative fluid balances
| Mean urine output [ml] (SD) |
Mean blood loss [ml] (SD) |
Mean non-study related fluid replacement [ml] (SD) |
|
| Mannitol group |
1869 (± 914) |
1244 (± 792) |
8608 (± 2289) |
| Placebo group |
886 (± 530) |
950 (± 672) |
8569 (± 2754) |
| P-value |
0.0 |
0.16 |
0.96 |
Table 3. Comparison of the mean dose of epinephrine and phenylephrine delivered to the
mannitol and placebo group.
| Mannitol group (n=24) |
Placebo group (n=26) |
P-value |
Mannitol group receiving epinephrine (n=9) / phenylephrine (n=12) |
Placebo group receiving epinephrine (n=12) / phenylephrine (n=14) |
P-value |
|
| Mean epinephrine dose (μg) |
23.8 |
51.6 |
0.177 |
63.6 |
111.8 |
0.20 |
| Mean phenylephrine dose (μg) |
355.1 |
992.3 |
0.056 |
710.3 |
1842.8 |
0.03 |
| Mean delivery time of epinephrine (min) |
6.88 |
12.7 |
0.216 |
18.3 |
27.5 |
0.24 |
| Mean delivery time of phenylephrine (min) |
10 |
17.7 |
0.181 |
20 |
32.9 |
0.12 |
Figure 1. Averages of mean arterial blood pressures for mannitol and placebo
(0.9% NACL) group
Addresses of authors:
1) University of Washington Medical Center 2) University of Washington
Department of Anesthesiology Department of Biostatistics
P.O. Box 356 540 P.O. Box 357 232
1959 NE Pacific Street 1959 NE Pacific Street
SEATTLE SEATTLE
Washington 98195-6540 Washington 98195-6540
U.S.A. U.S.A.
Address for correspondence:
Greg Dembo, M.D. Phone: (206) 598-4260
University of Washington Medical Center Fax : (206) 598-4544
Department of Anesthesiology E-mail: gdembo@u.washington.edu
P.O. Box 356 540
1959 NE Pacific Street
SEATTLE
Washington 98195-6540
U.S.A.
All pages copyright ©Priory Lodge Education Ltd 1994-2006.