Browse through our Journals...
EARLY EXTUBATION AND WEANING WITH BILEVEL POSITIVE AIRWAY PRESSURE VENTILATION AFTER CARDIAC SURGERY
Kilic et al - author details
Key words: BiPAP , coronary artery bypass,early extubation
Abstract
Purpose: To evaluate the use of bilevel positive airway pressure (BiPAP)ventilation in early extubation after fast-track cardiac surgery.
Methods: Sixty consecutive patients eligible for early extubation after cardiac surgery were randomly assigned to pressure support-continuous positive airway pressure or BiPAP (Group I or Group II, respectively) and were extubated.
Blood gases and hemodynamics were determined on arrival in the ICU (baseline) and 1, 2, 4, 6, 8, and 12 hours later. All data were expressed as (±SD) and analyzed using the Student t-test and Mann-Whitney test (continuous data) or χ2 test . P < 0.05 was considered statistically significant.
Results: PaCO2 levels were statistically significantly higher in Group II than in Group I at 2, 4, 6, 8, and 12 hours (P<0.05, P<0.01). Within each group, PaCO2 levels were statistically significantly higher at 4, 6, 8, and 12 hours than at baseline (P<0.01 ). Extubation time was significantly longer in Group I than in Group II 7.90 (7.90 [2.13] vs 3.83 [1.20], P=0.001). Respiratory rates were significantly higher in Group II than in Group I after 2, 4, and 6 hours (P<0.01).
Conclusions: Early extubation and weaning to BiPAP ventilation after cardiac surgery is safe and effective.
Introduction
Improving outcomes while reducing costs is a significant challenge in the postoperative care of cardiac surgery patients. Rapid (“fast-track”) protocols designed to shorten length of stay (LOS) after cardiac surgery have become common place. A key component of such protocols is extubation of the patient early after arrival in the surgical intensive care unit (ICU). As surgical and anesthetic techniques have advanced, extubation within 4 to 6 hours after coronary artery bypass graft (CABG) surgery has become a preferred goal at many institutions. Nevertheless, how early to extubate remains a matter of debate (1).
There is ample evidence that early extubation after cardiac surgery is safe and well tolerated, provided that patient selection and surgical, anesthetic, and postoperative management are carefully and diligently performed. Meanwhile, the role of respiratory management in successful early extubation has received little attention. Several clinical studies have aimed to identify the best approach to rapid postoperative weaning of patients from respiratory support (2).
Cardiac surgery patients at our hospital are extubated within the first 3 hours after admission to the ICU, according to a fast-track protocol. This protocol is followed whenever possible, even in some cases when a patient’s spontaneous breathing is inadequate. The immediate aim is to lower the risk of early postoperative complications such as hemodynamic instability, hypermetabolism, shivering, and mediastinal bleeding.
One potentially useful means of weaning patients from respiratory support is bilevel positive airway pressure (BiPAP). This noninvasive mode of ventilatory support combines pressure-controlled ventilation and spontaneous breathing to create 2 different positive airway pressure levels—inspiratory (IPAP) and expiratory (EPAP) (3). These 2 levels of pressure change in response to the patient's respiratory cycle (3). Studies performed in the mid to late 1990s demonstrated that BiPAP ventilation is safe and effective in patients with many types of respiratory failure (4), in patients with chronic congestive heart failure (5), and in patients at high risk of postoperative complications (6). More recent studies have shown that BiPAP ventilation is safe and effective in mechanically ventilated patients who do not require total ventilatory support, even in the face of differences in initiating and maintaining the inspiratory and expiratory phases, in breathing circuits, and in interventilator circuits (7). However, our search of the literature revealed no reports on the postoperative use of BiPAP to early weaning of cardiac surgery patients from ventilatory support. The aim of the present study was to evaluate the utility of BiPAP ventilation in achieving early extubation of eligible patients after cardiac surgery.
Methods
Study was designed as a prospective observational study.Between May 2005-December 2005 sixty patients were included in study, eligible for early extubation after cardiac surgery. This study was approved by an institutional ethics committee, and all patients gave informed consent before participating. Only patients undergoing CABG surgery were eligible for inclusion in the study .Exclusion criteria were hypothermic circulatory arrest, recent myocardial infarction, aortic and mitral valve problems, and perioperative intra-aortic balloon pump insertion.
After enrollment and before undergoing CABG surgery, each patient was randomly assigned to one of 2 modes of ventilatory support: pressure support-continuous positive airway pressure (PS-CPAP) (Group I) or BiPAP (Group II). All patients were instructed in the purpose and use of both ventilatory support modes.
Preoperatively, all patients received diazepam by orally the night before surgery and intramuscular midazolam (0.5–1 mg/kg) scopolamine (0.5 mg) 1 hour before the induction of anesthesia. Patient vital signs and other safety parameters were monitored by means of 5-lead electrocardiography, pulse oximetry, capnography, invasive measurement of radial artery pressure, and measurement of central venous pressure via the right internal jugular vein. Electrocardiographic leads, a pulse oximeter, and an arterial line were put in place on the patient’s arrival in the operating room. A central venous catheter was inserted via an 8F introducer (Maxxim Medical Inc.) after induction of anesthesia. Ventilation was achieved by maintaining a tidal volume of 10 mL/kg and adjusting the respiratory rate to an end-tidal carbon dioxide level of 30-34 mmHg. Positive end-expiratory pressure (PEEP) was not used. Anesthesia was induced and maintained by infusion of fentanyl (10 µg/kg) and propofol (2 mg/kg). Tracheal intubation was facilitated by intravenous bolus administration of rocuronium bromide (0.8 mg/kg). After intubation, intermittent positive pressure ventilation (IPPV) was initiated to achieve an inspired oxygen fraction (FiO2) of 1, a respiratory rate of 12 breaths per minute, and a peak inspiratory pressure (Pmax) of 30 mmHg. Anaesthesia was maintained by infusion of fentanyl (8 mcg/kg/h), propofol (2 mg/kg/h), and rocuronium bromide (8 mcg/kg/min). The infusion of neuromuscular agents was terminated 1 hour before the completion of surgery. No additional neuromuscular blocking was needed after termination of CPB.
Persistent perioperative systemic hypertension (systolic blood pressure >140 mmHg) was treated with infusions of nitroglycerin and nitroprusside, which were titrated to achieve a systolic arterial blood pressure of 80-100 mmHg. Mean perfusion pressure was maintained at 50-70 mmHg. In all cases, CABG surgery was performed while the patient was receiving moderate hypothermic CPB support (minimal rectal temperature of 33.7 ± 1.6°C); after surgery, CPB was terminated only after the patient was actively rewarmed to a body (rectal) temperature of 37°C .
Upon arrival in the intensive care unit (ICU) after surgery, patients in both study groups were started on mechanical ventilation provided by a Servo 900C ventilator (Siemens Elema, AB, Sweden). Initially, the ventilator was set to operate in simultaneous intermittent mandatory ventilation (SIMV) mode to provide a tidal volume of 10 ml · kg-1, a respiratory rate of 12-15 breaths per minute, a PEEP of 5 cm H2O, and a FiO2 of 1.0. Trigger sensitivity was set at -2 cm H2O. The FiO2 was reduced to 0.4 or less on the basis of blood gas levels and oxygen saturation as monitored by pulse oximetry (SpO2). When spontaneous breathing reappeared, patients were treated in one of 2 ways. Each patient in Group I was switched to PS-CPAP mode at a pressure support level of 10 cm H2O. Each patient in Group II had his or her endotracheal tube connected to a BiPAP ventilator operating in ST mode (BiPAP Vision, Respironics Inc., Murrysville, PA) at a preset inspiratory pressure of 15 cm H2O and a preset expiratory pressure of 5 cm H2O. In this way, the BiPAP ventilator was able to respond to the patient's respiratory cycle by alternating between a higher flow rate during inhalation and a lower flow rate during exhalation. The back-up flow rate was set at 12 breaths per minute. The circuit of a standard BiPAP S/T-D ventilator consists of a single limb for expiration and inspiration; oxygen was obtained via the central oxygen line
Weaning from ventilatory support was achieved as follows. Patients in Group I were weaned while in CPAP mode by reducing the level of PS to 5 cm H2O as tolerated. Once this level of support was achieved, patients were extubated and received nasal oxygen therapy. Patients in Group II were weaned by reducing IPAP to 10 cm H2O and EPAP to 5 cm H2O. After extubation, these patients underwent extubation, and BiPAP ventilatory support was continued through a special full-face mask.
After extubation, oxygen saturation was monitored by a pulse oximetry (SpO2). Humidification was provided by means of a Filta-Therm heat and moisture exchanging filter (Intersurgical Inc., Liverpool, NY). Fentanyl was infused intravenously (1 mcg/kg/h) to provide analgesia during 1st postoperative hour. Meperidin (25 mg) was injected intramuscularly to prevent shivering .A bolus of tenoxikam (20 mg) was administered intravenously 30 minutes before extubation.
In the ICU, hypotension (ie, mean arterial pressure below 50 mmHg) was treated with crystalloids and colloids or with ephedrin boluses. Tranfusions were used as necessary to maintain hematocrit at a value greater than 25%. Patients in the ICU were extubated only if they met the following criteria:
- Awake,cooperative,and no residual neoromuscular block
- Partial pressure of oxygen (PaO2 )>80 mmHg (FiO2 = 0.4)
- Partial pressure of carbondioxyde (PaCO2 )<45 mmHg
- Stable hemodynamic and metabolic parameters
- Bleeding <50 mL/hour
- No shivering
Blood gas and lactate levels were measured and analyzed .The oxygenation index (PaO2/FiO2) was calculated and recorded. Hemodynamics were measured and blood samples were taken at the following predetermined time points: on arrival in the ICU (T0) and 1, 2, 4, 6, 8, and 12 hours postoperatively.
All data were expressed as standart deviation (SD). Statistical analyses were performed using SPSS for Windows 10.0. Data were compared between groups using the Student t-test and Mann-Whitney test. Categorical data were compared against a chi-square distribution. A P value of less than 0.05 was considered to be statistically significant.
Results
Overall, the study population included 60 consecutive patients (42 men [70%] and 18 women [30%]) undergoing elective CABG surgery with CPB support. The mean (SD) patient age was 54.62 ± 10.66 years.
Both study groups were demographically similar (Table 1).
Table 1: Patient Demographics and Characteristics (n=60)
Characteristic |
GroupI (PS-CPAP) |
GroupII (BiPAP) |
|
|
n (%) |
|
|
Gender |
|
|
|
Men |
23 (76.7) |
19 (63.3) |
χ2=1.270 P=0.260 |
Women |
7 (23.3) |
11 (36.7) |
|
Mean (SD) |
|
||
Age (years) |
57.63 (12.02) |
52.43 (8.42) |
P=0.057 |
Weight (kg) |
77.47 (9.17) |
82.33 (11.07) |
P=0.069 |
|
n (%) |
|
|
Medical history |
|
|
|
Diabetes mellitus |
6 (20.0) |
8 (26.7) |
χ2=0.37 P=0.542 |
Heart transplant |
5 (16.7) |
18 (60.0) |
χ2=11.915 P=0.001* |
Myocardial infarction |
14 (46.7) |
14 (46.7) |
χ2=0.000 P=1.000 |
t: Student t test χ2: chi-square test
* P<0.01.
Clinically, they were also similar in terms of mean arterial pressure (MAP) (Table 2), heart rate (Table 3), total bypass and cross-clamp times (Table 4), and inotrope use (Table 5).
Table 2: Mean Arterial Pressure
MAP (mmHg)a |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
92.33 (14.42) |
91.67 (10.49) |
0.839 |
1 h |
86.73 (18.02) |
89.10 (15.34) |
0.586 |
2 h |
85.60† (12.13) |
82.97† (13.88) |
0.437 |
4 h |
82.87†† (11.24) |
85.50† (12.33) |
0.391 |
6 h |
83.23†† (14.87) |
81.53†† (14.23) |
0.653 |
8 h |
83.17†† (11.66) |
86.13† (11.17) |
0.318 |
12 h |
82.13†† (8.13) |
81.87†† (12.23) |
0.921 |
aValues shown are mean (SD).
† In group P<0.05
†† In group P<0.01
However, MAP did fall slightly in both groups (Table 2) and heart rate increased in Group II (Table 3, Figure 1) after 4 hours in the ICU.
Table 3: Heart Rate
Heart rate (bpm)a |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
86.63 (20.91) |
82.70 (14.08) |
0.396 |
1 h |
96.23†† (24.56) |
88.57† (15.83) |
0.156 |
2 h |
95.90†† (20.91) |
89.83†† (12.72) |
0.180 |
4 h |
93.60† (18.99) |
97.03†† (17.16) |
0.466 |
6 h |
95.33†† (16.34) |
87.00 (17.32) |
0.060 |
8 h |
91.43 (16.81) |
88.23 (15.36) |
0.445 |
12 h |
89.47 (14.96) |
88.43 (15.92) |
0.796 |
aValues shown are mean (SD).
† In group P<0.05
†† In group P<0.01
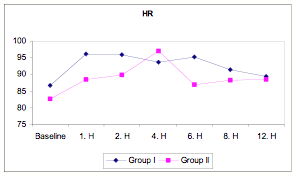
Figure 1
Figure 1: Heart rate in BiPAP versus PS-CPAP patients.
Table 4: Mean Total Bypass and Cross-clamp Times
Time (min)a |
P value |
||
GroupI (SIMV-CPAP) |
GroupII (SIMV-BiPAP) |
||
Total bypass |
88.70 (32.99) |
85.29 (14.17) |
0.679 |
Cross-clamp |
55.25 (25.62) |
60.06 (13.18) |
0.469 |
aValues shown are mean (SD).
Table 5: Extubation Times and ?notropic Agent Usage
|
GroupI (SIMV-CPAP) |
GroupII (SIMV-BiPAP) |
|
Mean (SD) |
|
||
Time to extubation (h) |
7.90 (2.13) |
3.83 (1.20) |
P=0.001** |
|
n (%) |
|
|
Inotrope (mcg/kg/dk) |
|
||
None |
24 (80.0) |
29 (96.7) |
χ2=4.272 P=0.118 |
Dopamine |
2 (6.7) |
-- |
|
Dobutamine |
4 (13.3) |
1 (3.3) |
|
t: Student t-test; χ2: chi-square test.
*P<0.05
**P<0.01
There were several significant differences between the groups. PaCO2 levels were statistically significantly higher in Group II than in Group I at 2, 4, 6, 8, and 12 hours (P<0.05, P<0.01). Within both groups, PaCO2 levels were statistically significantly higher at 4, 6, 8, and 12 hours than at baseline (P<0.01 ) (Table 6, Figure 2).
Table 6: PCO2
PCO2 (mmHg)a |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
31.33 (5.26) |
33.09 (4.47) |
0.07 |
1 h |
30.42 (4.88) |
33.17 (6.94) |
0.08 |
2 h |
31.50 (4.83) |
35.90 (7.20) |
0.007** |
4 h |
33.82†† (4.96) |
42.29†† (7.06) |
0.001** |
6 h |
34.15†† (6.23) |
40.67†† (4.04) |
0.001** |
8 h |
36.23†† (4.97) |
39.52†† (4.95) |
0.013* |
12 h |
35.56†† (4.40) |
39.31†† (4.34) |
0.002** |
t: Student t-test.
aValues shown are mean (SD).
* P<0.05
** P<0.01
† In group P<0.05
†† In group P<0.01
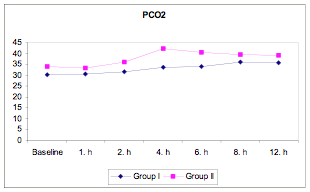
Figure 2
Figure 2: PaCO2 in BiPAP versus PS-CPAP patients
Table 7: Respiratory Rate
Respiratory rate (breaths per minute)a |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
11.83(0.46) |
11.97(0.18) |
0.146 |
1 h |
13.20(3.99) |
14.20†(5.95) |
0.448 |
2 h |
12.60†(1.97) |
17.83††(5.05) |
0.001* |
4 h |
13.00††(2.26) |
17.93††(3.95) |
0.001* |
6 h |
13.93††(2.93) |
18.21††(3.37) |
0.001* |
8 h |
16.00†† (3.74) |
16.69†† (2.59) |
0.416 |
12 h |
17.28†† (2.90) |
16.38†† (3.10) |
0.260 |
t: Student t-test
aValues shown are mean (SD).
* P<0.01
† In group P<0.05
†† In group P<0.01
Table 8: Peak Inspiratory Pressure
Pmax (mmHg)a |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
24.62 (4.10) |
23.85 (3.88) |
0.458 |
1 h |
24.53(4.16) |
22.00 (4.07) |
0.026* |
2 h |
24.18(4.09) |
25.00 (4.79) |
0.649 |
4 h |
23.14††(4.06) |
-- |
|
6 h |
20.75††(3.82) |
-- |
|
8 h |
21.22††(3.86) |
-- |
|
12 h |
19.85(1.63) |
-- |
|
Pmax: peak inspiratory pressure; t: Student t-test
aValues shown are mean (SD).
*P<0.05
†† In group P<0.01
Table 9: Work of Breathing
WOBa |
P value |
||
GroupI (PS-CPAP) |
GroupII (BiPAP) |
||
Baseline |
1.48 (0.13) |
1.51 (0.14) |
0.294 |
1 h |
1.28†† (0.13) |
1.25†† (0.12) |
0.457 |
2 h |
1.22†† (0.32) |
1.09†† (0.10) |
0.294 |
4 h |
3.03 (11.51) |
-- |
-- |
6 h |
3.63 (14.76) |
-- |
-- |
WOB: work of breathing; t: Student t-test
aValues shown are mean (SD).
Extubation time was significantly longer in Group I than in Group II (Figure 3).
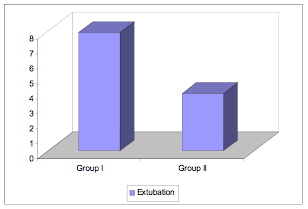
Figure 3
Figure 3: Extubation times in BiPAP versus PS-CPAP patients.
Respiratory rates were similar between groups at baseline and at 1, 2, 8, and 12 hours postoperatively (P>0.05) but were significantly higher in Group II than in Group I at 2, 4, and 6 hours postoperatively (P<0.01). Within both groups, respiratory rates were higher at all time points than they were at baseline (P<0.05, P<0.01) (Table 7, Figure 4).
One hour postoperatively, Pmax was higher in Group I than in Group II. At baseline and 2 hours postoperatively, Pmax was similar between groups. Four hours postoperatively, no between-group comparison of Pmax values was possible because patients in Group II were extubated at that time point. At 4, 6, 8, and 12 hours postoperatively, Pmax values declined progressively in Group I (Table 8).
There was no significant difference in work of breathing (WOB) between groups at 1 and 2 hours postoperatively (P>0.05). At 4 and 6 hours postoperatively, between-group comparisons of WOB were impossible, again because patients in Group II were extubated at those time points (Table 9).
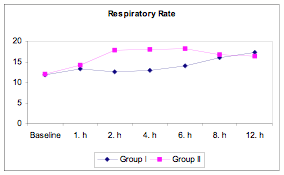
Figure 4
Figure 4: Respiratory rate in BiPAP versus PS-CPAP patients..
Discussion
In the era of managed care, much emphasis has been placed on reducing the length of ICU stays after CABG surgery. Rapid (fast-track) weaning protocols have become increasingly popular because of their apparent cost-effectiveness and safety (8). However, the risk of respiratory complications after cardiac surgery remains high, mainly because of the complications classically associated with thoracic surgery, tracheal intubation, mechanical ventilation, and other factors such as age.
In cardiac surgery, prolonged controlled ventilation has long been standard practice, mainly because such surgery requires intraoperative high-dose narcotic anesthesia and is associated with an increased risk of myocardial ischemia in the early postoperative period. However, fast-track extubation (FTE) protocols are becoming increasingly popular mainly due to a greater rationalization of resource utilization.
Recent well-documented data indicate that early extubation (ie, less than 8 hours) after cardiac surgery may be both clinically effective and cost effective. Reductions in ICU and overall hospital LOS are associated with lower costs in the postoperative period. However, the best time for early extubation remains open to debate, and the safety of very early or immediate extubation just after surgery remains controversial.
Postoperative ventilation and intubation times are influenced by several factors. In a study by Reis and colleagues (9), in which patients were extubated immediately after certain criteria were achieved (ie, return to consciousness, normothermia, stable hemodynamics, acceptable respiratory function, and hemostasis), the researchers concluded that the success of FTE depended strongly on postoperative monitoring, control of weaning from mechanical ventilation, and criteria-based endotracheal extubation (9).
Though safe, tracheal intubation and mechanical ventilation are not entirely benign. Endotracheal intubation contributes to morbidity, especially as the duration of intubation increases. Approximately two thirds of patients intubated for more than 24 hours show signs of vocal cord granulomas and ulcerations. After 1 hour of application, an inflated, cuffed endotracheal tube significantly depresses the velocity of tracheal mucus. Postpoperatively, complications including pneumonia, atelectasis, lobar collapse, increased total body water, and arterial blood gas deterioration occur more frequently in patients extubated more than 24 hours postoperatively than they do in patients extubated earlier. Higgins described a “window of opportunity” between the 3rd and 10th hours after ICU admission during which cardiac surgery patients may be optimally extubated. He also noted that extubation should be preceded by a thorough physiologic and clinical evaluation in which careful attention is paid to hemodynamics, neurologic status, temperature and metabolism, hemostasis, and respiratory reserve (10).
In a 30-day postoperative follow-up study of cardiac surgery patients, Cheng et al. showed that early extubation diminished the incidence of intrapulmonary shunting but had no effect on postoperative mortality (11). They also demonstrated that early tracheal extubation and anesthetic management could reduce the total cost of CABG surgery by 25% (11).
In our present study, the mean (SD) time to extubation was 7.90 ± 2.13 hours in Group I versus 3.83 ± 1.20 hours in Group II. We hypothesize that the time to extubation in Group II was likely shorter because the BiPAP ventilator provides a more physiologic,unrestricted breathing pattern and has a more sensitive trigger mechanism. Thus, the FTE criterion of spontaneous breathing would have been met earlier in the BiPAPsupported group.
Weaning patients from mechanical ventilation may be problematic and difficult in the face of imposed work of breathing (WOBi). In performing the WOBi, the patient can trigger BiPAP ventilation and thus overcome the additional flow-resistant effects of the circuit and the endotracheal or tracheotomy tube. Indeed, WOBi is a recognized potential cause of weaning failure.
Nevertheless, the clinical significance of WOBi remains controversial. Its effect on the ability to wean patients from ventilatory support postoperatively may not be clinically significant in stable patients whose cardiopulmonary function is good, but it may be clinically significant in patients whose cardiopulmonary reserve is marginal and who may thus be kept unnecessarily ventilated. Several groups have implicated WOBi as a cause of failure to wean; others have argued that the work of breathing increases after extubation, possibly secondary to upper airway edema, and that WOBi merely offsets this increase in work after extubation (12).
In the present study, we observed no significant difference in WOB between our 2 study groups. In Group II, we avoided extubating any patient earlier than 3 hours after surgery in light of chest tube drainage and ventricular dysfunction. Even if a patient’s spontaneous breathing was sufficient, the patient was connected to the BiPAP ventilator via his or her endotracheal tube, and no further adjustments to ventilator settings were made. In this way, we also aimed to reduce the patient’s WOB. Ishaaya et al. reported that the mean WOB and pressure time product were significantly greater after extubation than during spontaneous breathing through an endotracheal tube (13). In the present study, we could not measure WOB after extubation (ie, after 4 hours in the ICU).
In a prospective study, Kazmaier and colleagues compared the influences of BiPAP and CPAP on systemic hemodynamics and ventilatory parameters and found that BiPAP-ventilated patients, in contrast to CPAP-ventilated patients, were able to breathe spontaneously independently of the mechanical ventilatory cycle. Kazmaier’s group concluded that the negative hemodynamic effects of BiPAP do not counterbalance its beneficial properties. They also found that Pmax differed between the groups during the period of sedation (ie, increased in the CPAP ventilated patients), but that hemodynamic parameters did not (14).
In the present study, we too observed several notable differences between our CPAP- and BiPAP-ventilated groups. First, Pmax was greater in Group I than in Group II at T1. Second, respiratory rate, PCO2, PO2, and PH differed significantly between the 2 groups at various time points. In particular, PCO2 was significantly greater in Group II than Group I at all time points. We considered these differences to be due to the conditions imposed by the BiPAP machinery, which are similar to physiological conditions. Having a high respiratory rate 2, 4, and 6 hours postoperatively allowed extubation criteria to be reached more quickly. Conversely, there was no difference between groups either in the respiratory rate at 8 and 12 hours postoperatively . Although there were statistically significant differences between groups in terms of base deficit and lactate levels, these differences were not considered clinically significant because all values fell within normal limits.
With the lone exception of heart rate, all other hemodynamic parameters were similar between groups. Heart rate was significantly higher in Group II than in Group I 2 and 4 hours postoperatively. It is possible, however, that the significant increase in heart rate in Group II patients at these 2 time points could have been due to excitation and manipulation of the heart during extubation.
Although there was a difference in the timing of extubation between groups, there was no difference in the timing of surgery, ICU LOS, or overall hospital LOS. The mean time to extubation postoperatively was twice as long in Group I than in Group II (7.9 ± 2.13 hours versus 3.83 ± 1.20 hours. The similarity in ICU and overall hospital LOS between groups was due to our surgery team’s protocol for discharge.
After CABG surgery using hypothermic CPB, a prolonged period of analgesia and sedation is usually preferred to reduce stress-induced sympathetic activation. This is intended to decrease the risk of early postoperative myocardial ischemia. Rathgeber and colleagues demonstrated that the type of ventilatory support can influence postoperative demand for analgesics and sedatives and that the application of BiPAP ventilation or SIMV instead of controlled mechanical ventilation can significantly decrease such demand. This suggests that BiPAP may be preferable for both short-term and long-term ventilation of patients after CABG surgery (15).
In our study population, the infusion of fentanyl 1mcg/kg/hour as analgesic therapy and administered tenoxicam as a bolus IV 30 min before extubation provided sufficient analgesia. There was no significant difference between the groups with respect to analgesic requirements, and neither group required additional analgesia.
In recent years, many groups have sought to develop new modes of weaning CABG patients from ventilatory support postoperatively in the ICU. The use of noninvasive ventilators for weaning has gained in popularity. For example, noninvasive positive pressure ventilation (NIPPV), which is primarily used to avoid intubation, has also been used to facilitate weaning. The pooled results from 2 prospective, randomized, controlled trials suggest that weaning via extubation to NPPV can reduce the duration of mechanical ventilation, ICU stays, mortality, and incidence of nosocomial pneumonia (16, 17).
So far, there appears to be only one study reported in the literature to have examined the effects of BiPAP ventilation on intubated patients. In brief, Patel et al. compared the effects of BiPAP S/T versus conventional ventilation on respiratory muscle performance, pulmonary mechanics, and gas exchange. They concluded that BiPAP S/T is safe and effective when used in patients who need mechanical ventilation via endotracheal tube or tracheostomy (7).
Traditionally, the use of BiPAP in cardiac surgery has been limited to its use as a mode of conventional ventilation or as a means of providing respiratory support while avoiding reintubation. In studies performed by Eren and colleagues, 15 patients who underwent open heart surgery under CPB were weaned via extubation to NIPPV in the ICU (18). Five of these patients had to be reintubated after a relatively brief period of NIPPV support (mean [SD], 3 ± 0.5 hours) because of the reoccurrence of hypoxia, hypercapnia, and impaired hemodynamics. The other 10 patients experienced improvement in respiratory function and so were able to continue on NIPPV and avoid reintubation. One of these 10 patient, however, was re-entubated (18).
In other studies, Takami and colleagues demonstrated the useful effects of BiPAP in cardiac surgery patients (19). Theoretically, as Takami et al. noted, EPAP provides physiological advantages similar to those of PEEP in mechanical ventilation, and IPAP creates further PS effects that permit passive ventilation. These 2 pressure levels, alternating at preset time intervals, improve lung mechanics by recruiting atelectatic alveoli, increasing pulmonary compliance, and reducing the WOB. As a result, in Takami’s study, BiPAP ventilation not only increased the PaO2/FiO2 ratio in patients who were poorly oxygenated on arrival in ICU immediately after surgery and before extubation, but also allowed postextubation PaO2/FiO2 levels in these patients to be maintained at levels similar to those in non–BiPAP-supported patients.
BiPAP ventilation offers several potential advantages to patients. First, it ameliorates alveolar recruitment during both inhalation and exhalation. Second, CPAP increases oxygen uptake in the setting of impaired oxygen diffusion, which may result from acute lung injury during CPB. Third, by improving oxygenation, it may also increase tissue partial pressure of oxygen and thus promote wound healing and avoid surgical wound infection. Fourth, BiPAP ventilation, and especially its IPAP component, eases the WOB, which may in turn increase myocardial oxygen demand. Fifth, BiPAP ventilation may avoid the morbidities associated with endotracheal intubation for mechanical ventilation such as ventilator-associated pneumonia, patient discomfort, absence of oral intake and speech, and need for deep sedation. Sixth, BiPAP ventilation may improve hemodynamics by reducing afterload, transmural pressure, sympathetic nerve activity, and afterload, resulting in enhanced ventricular performance (19).
BiPAP ventilation is not appropriate for all patients who have undergone cardiac surgery with CPB. It is not appropriate for patients whose postoperative hemodynamics are unstable since stable hemodynamics is the major prerequisite for initiation of BiPAP support. It is also not appropriate in patients experiencing excessive airway secretion since, once it is in place, the BiPAP equipment allows no direct access to remove secretions.
In general, complications associated with BiPAP ventilation are uncommon. Gastric insufflation is rare at pressures of less than 30 cm H2O. The incidence of aspiration of gastric contents is very low (5% or less) when the airway reflex is intact. Local complications such as skin abrasion, sinus complaints, and conjunctivitis are more common. In our study population, there were no complications associated with BiPAP ventilation.
In conclusion, our study demonstrates that fast-track extubation and weaning to BiPAP ventilation after CABG surgery is safe and effective. BiPAP ventilation offers clinical benefits that warrant its earlier institution during the postoperative management of patients who have undergone cardiac surgery with CPB. In addition, our study suggests that BiPAP ventilation can be used safely not only for short-term support in patients who have undergone hypothermic CPB, but also for long-term support during periods of postoperative pulmonary dysfunction.
References
1. Konstantakos AK, Lee JH. Optimizing timing of early extubation in coronary artery bypass surgery patients. Ann Thorac Surg 2000;69:1842-1845.
2. Cassina T, Chiolero R, Mauri R, Revelly JP. Clinical experience with adaptive support ventilation for fast-track cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17(5):571-575.
3. Kosowsky JM, Storrow AB, Carleton SC: Continuous and bilevel positive airway pressure in the treatment of cardiogenic pulmonary edema. Am J Emerg Med 2000,18:91-95.
4. Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998;339:429 435.
5. Lin M, Yang YF, Chiang HT, Chang MS, Chiang BN, Cheitlin MD. Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema: shortterm results and long-term follow-up. Chest 1995;107:1379-1386.
6. Kindgen-Milles D, Buhl R, Gabriel A, Bohner H, Muller E. Nasal continuous positive airway pressure: a method to avoid endotracheal reintubation in postoperative high-risk patients with severe non hypercapnic oxygenation failure. Chest 2000;117:1106-1111.
7. Patel RG, Petrini MF. Respiratory muscle performance, pulmonary mechanics, and gas exchange between the BiPAP S/T-D System and the Servo Ventilator 900C with bilevel positive airway pressure ventilation following gradual pressure support weaning. Chest 1998;114(5):1390-1396.
8. Liu LL, Gropper MA. Respiratory and hemodynamic management after cardiac surgery. Curr Treat Options Cardiovasc Med. 2002;4(2):161-169.
9. Reis J, Mota JC, Ponce P, Costa-Pereira A, Guerreiro M. Early extubation does not increase complication rates after coronary artery bypass graft surgery with cardiopulmonary bypass. Eur J Cardiothorac Surg 2002;21:1026-1030.
10. Higgins TL. Pro: early endotracheal extubation is preferable to late extubation in patients following coronary arter surgery. J Cardiothorac Vasc Anesth 1992;6(4):488-493.
11. Cheng DC. Early extubation after cardiac surgery decreases intensive care unit stay and cost. J Cardiothorac Vasc Anesth 1995;9(4):460-4.
12. French CJ, Bellomo R, Buckmaster J. Effect of ventilation equipment on ?mposed work of breathing: Critical Care and Resuscitation 2001;3:148-152.
13. Ishaaya AM, Nathan SD, Belman MJ: Work of breathing after extubation. Chest 1995;107(1):204-209.
14. Kazmaier S, Rathgeber J, Buhre W, Buscher H, Busch T, Mensching K, et al. Comparison of ventilatory and haemodynamic effects of BiPAP and S-IMV/PSV for postoperative short-term ventilation in patients after coronary artery bypass grafting
Eur J Anaesthesiol 2000;17:601-610.
15. Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BiPAP) on duration of intubation and consumption of analgesics and sedatives: a prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol 1997;14:576-582.
16. Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure. Am J Respir Crit Care Med 1999;160:86-92.
17. Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease: a randomized, controlled trial. Ann Intern Med 1998;128:721-728.
18. Eren NT, Ery?lmaz S, Akar R, Durdu S, Çorapç?o?lu T, Akal?n H. Noninvasive positive pressure ventilation after cardiac surgery. Journal of Ankara Medical School 2002; 24(3):113118.
19. Takami Y, Ina H. Beneficial effects of bilevel positive airway pressure after surgery under cardiopulmonary bypass. Interactive Cardiovascular and Thoracic Surgery 2003;2:156-159.
Author Details
Abdullah KILIC, Nihan YAPICI, Yesim BICER, Turkan CORUH, Arif TARHAN*,Fikri YAPICI*,Zuhal AYKAC
Siyami Ersek Thoracic and Cardiovascular Surgery Center, Anesthesiology and Reanimation , *Cardiovascular Surgery department/ Istanbul-Turkey
Corresponding author:
Nihan Yapici,MD
Atifbey Sokak Gokdeniz Sitesi,D blok No :17- 34662 Acibadem/?stanbul/TURKEY
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


