Browse through our Medical Journals...
An effective device for rumen cannulation in sheep
aAbdel-Fattah, M. *aSedeek, A. M. and bSuliman, A. I. A.
aBeni-Suef Univ., Fac.Vet. Med., Surgery, anesthesiology and radiology Dept.
bAnimal Production Research Institute, Dokki, Giza, Egypt.
Summary
Presently there is great need for ruminal cannulation in small ruminants either for investigation of digestion or collection of ruminal samples, and this could be performed by many types of cannulas and techniques. Unfortunately, these commercial cannulas and techniques are not sufficient for long-term studying of the ruminal environment as a result of many complications. Here we show fabrication of cannula for sheep and a new technique for its implantation. The device was adapted to allow sampling of entire ruminal contents via cannulas with different diameters, which tightly sealed within ruminal fistula to ensure cleaner, achieve easier nursing of operated animals, and maintain normal ruminal environment. The device was applied into the sheep by one-stage operation. It has been successfully used in rams for 16 months without complications. Accordingly, this will encourage researchers to perform long-term studies of ruminal environment in small ruminants.
Introduction
Fistulation and cannulation of the rumen is an integral part of the bovine and ovine nutritional studies even it is also recommended as conservative treatment in cases of chronic bloat (Anderson et al, 1976). Thus, a need for successful durable ruminal fistulation and cannulation technique is required for the research field as well as in the day to day practice (Schnautz, 1957 and Wakanker et al, 1980). Many factors complicate ruminal fistulation and cannulation by conventional methods; accordingly various modifications in the processing of ruminal cannulas as well as fistulation techniques had been recorded to minimize complications (Schnautz, 1957, Wayne et al, 1959, Komarek et al, 1961, El-Monzaly, 1975, Corley et al, 1999, and Nocek et al, 2002). A single-stage ruminal fistula technique was quite successful in sheep while two-stage technique was recommended in cattle (Dougherty, 1981 and Hassanein et al, 1988). However, several types of ruminal cannula and respective surgical techniques have been reported by Brown et al (1968) and Santra et al (2002). Ruminal cannulas were made of plastic, rubber, or even metal materials that should be placed and fixed properly to prevent leakage (Hecker, 1974, Thyfault et al, 1975, and Dougherty, 1981). Ruminal fistulation and cannulation was adopted practically for obtaining samples of ruminal ingesta, fluid, or gases (Schnautz, 1957, Komarek et al, 1961, and Corley et al, 1999) and fistulated animals maintained functioning satisfactorily for about 10 months (Nangeroni, 1954).
Materials and methods
Animal model: -
The study was performed on 11 healthy rams of native breed of 3-5 year-old, and ranged in weight between 40-65 kg.
Preoperative preparation: -
Food was withheld for 24 hours, while water was withheld for 12 hours, prior to surgery and the left para-lumber region was prepared for aseptic surgery. The operation was carried out in recumbent position and the operated animals were positioned on the right side.
Anesthesia: -
Operated animals were injected intravenously with Diazepam 0.5% in a dose of 2 mg/kg Bwt. and 10 minutes later, animals were injected, slowly intravenous, with thiopental sodium 2.5% in a dose of 10 mg/ kg Bwt. (Abdel-fattah, 1999).
Fabricated cannula: -
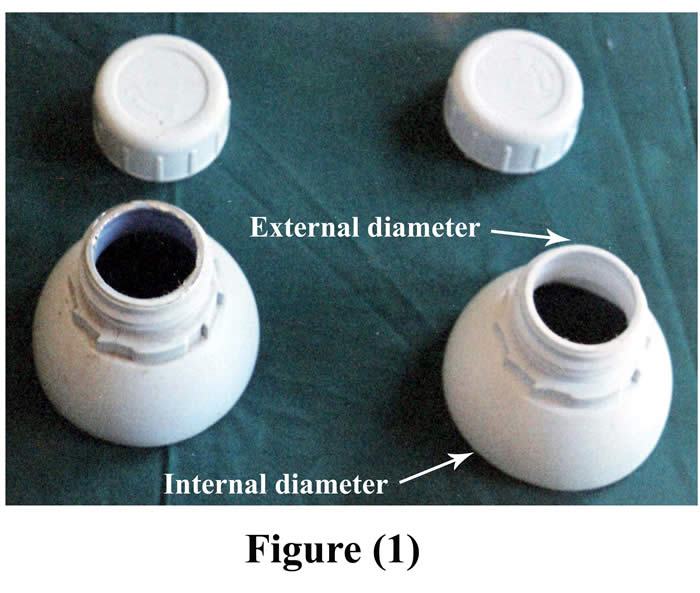
The cannula used in this technique was made of the upper part of polypropylene plastic bottle with its screwed cap, four pairs of small button-like plastic pieces, and strong non absorbable suture material (silk). The upper part of different sizes polypropylene plastic bottle was cut off in such way as to include the neck and part of the flange of the top of the bottle. This part of the bottle was stoppered by screwed cap. With respect to small cannula, diameter of external opening was 4 cm, diameter of internal opening was 7.5 cm, and its weight was 28 gm (Fig. 1).
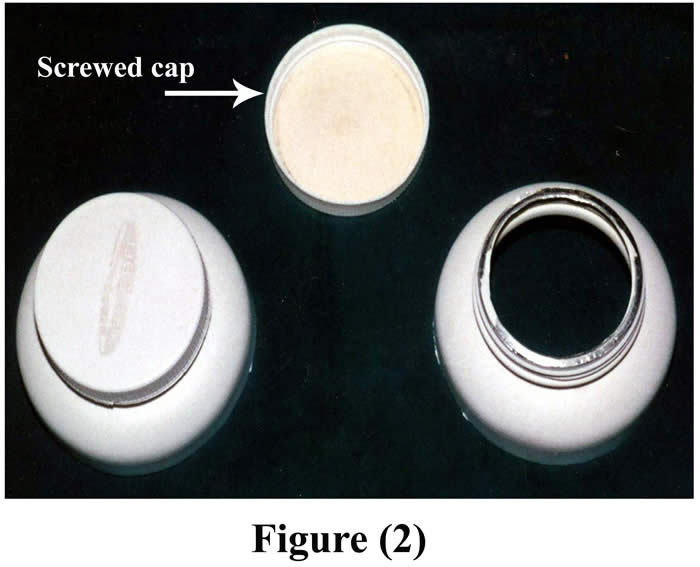
On the other hand, the diameter of the external opening of the large cannula was 7 cm, the diameter of the internal opening was 12 cm, and the weight of the cannula was 35 gm (Fig. 2).
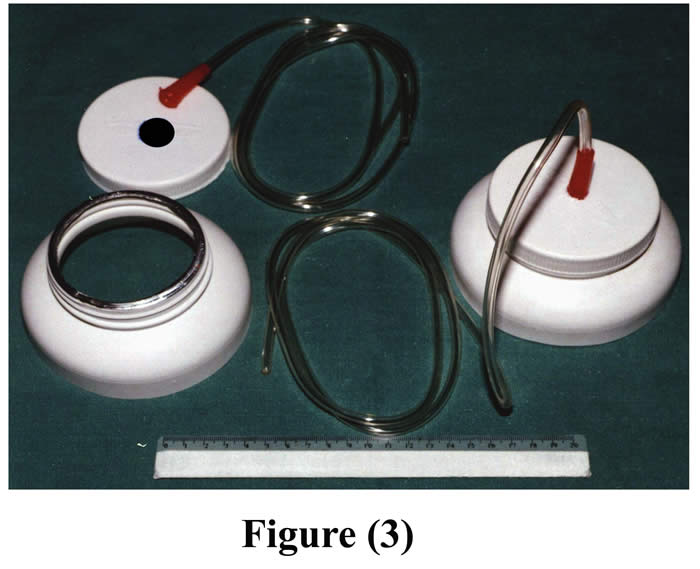
 The stopper of the cannula could be modified by fixing a rubber tube to its center for sampling of gases (Fig. 3).
The stopper of the cannula could be modified by fixing a rubber tube to its center for sampling of gases (Fig. 3).
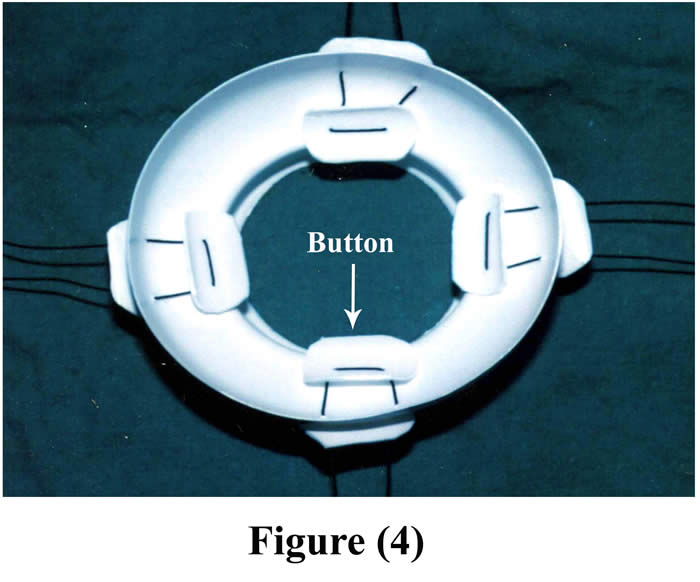
For good fixation, 2-4 button-like plastic pieces (according to the size of operated animal) were placed in the inner aspect of the main piece of the cannula and threaded by silk suture materials to another four plastic pieces out side the body. Figure (4) shows the final shape of the cannula.
Surgical technique: -
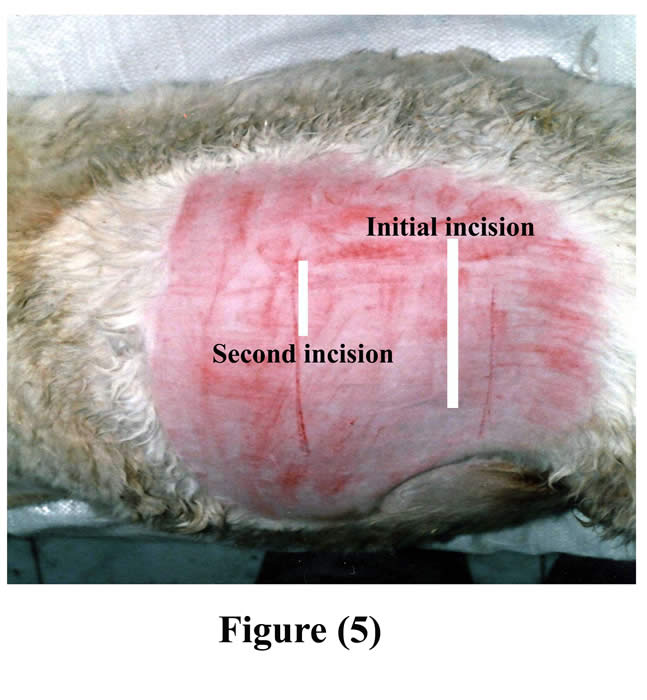
An initial skin incision was made few centimeters inferior to the tuber coxae and extended ventrally. The length of skin incision, as well as the underlying layers included the ruminal incision, was made large enough for insertion of the cannula (Fig 5).
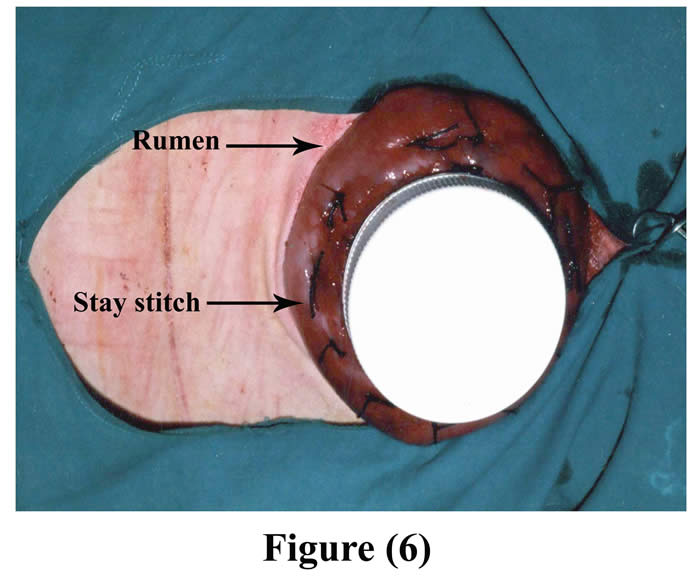
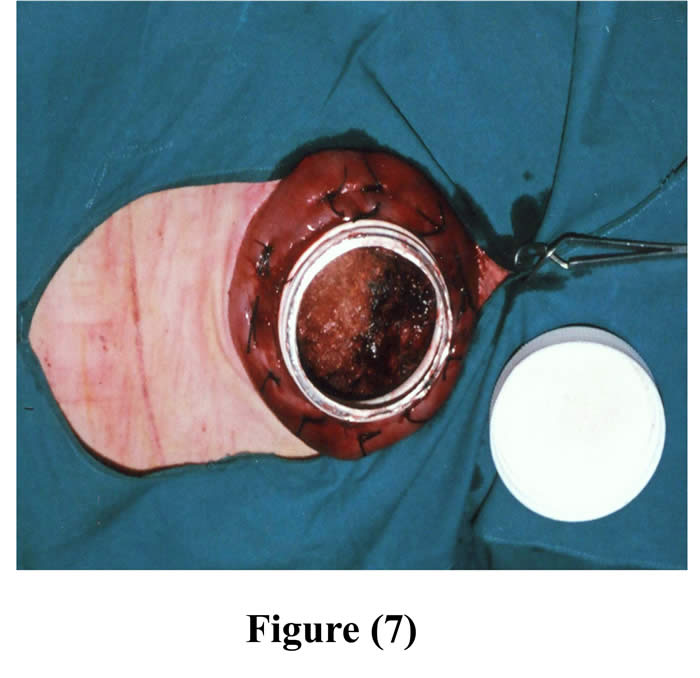
Another ruminal incision was made 7-10 cm cranial to the original one and created parley wide for the neck of the cannula to be exteriorized through it. The cannula was positioned into the rumen through the initial incision and pushed from inside the rumen through the second ruminal incision, and 4-6 interrupted sutures were stitched through the rumen and cannula by silk, then a purse string suture was sewed around the edge of the second ruminal incision and was drawn tight and tied around the neck of the cannula (Figs 6 & 7).
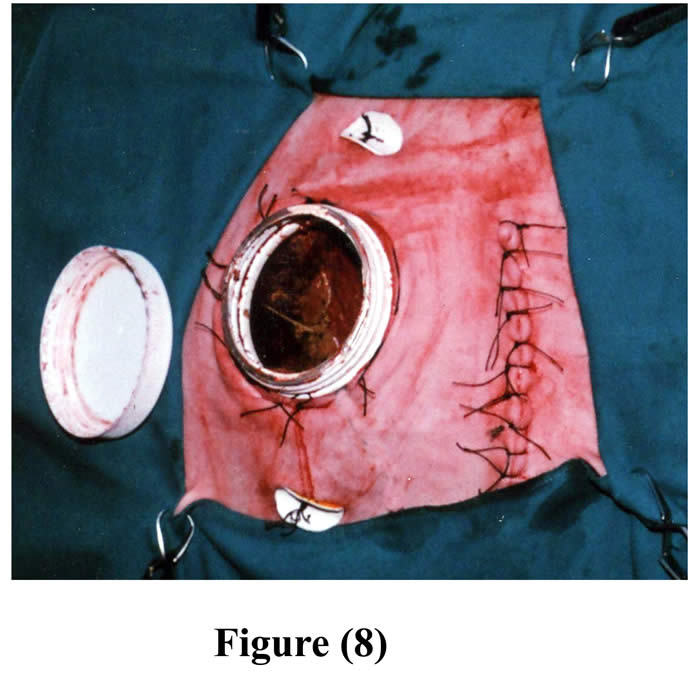
The initial ruminal incision was closed as usual and the rumen was repositioned into the peritoneal cavity. The skin and subcutaneous tissue, 7-10 cm cranial to the initial incision, were bluntly dissected from the abdominal muscles and a circular excision was made through the muscles and peritoneum. The excision in the muscles and peritoneum created a hernial ring that was wide enough to force the cannulated part of the rumen through it. The herniated part of the cannulated rumen with its cannula was forced through the hernial-like ring, and then continuously sutured around the contour of the cannula with the muscles and peritoneum. A second skin incision was made corresponding to outer opening of the cannula. This skin incision was created parley enough for the neck of the cannula to be forced through it. Then 4-6 staying stitches were made around the neck of the cannula, between skin and underlying herniated rumen (Fig 8).
A purse string suture was sewed around the edge of skin incision via subcutaneous layer and was drawn tight and tied around the neck of the cannula (Fig 9).
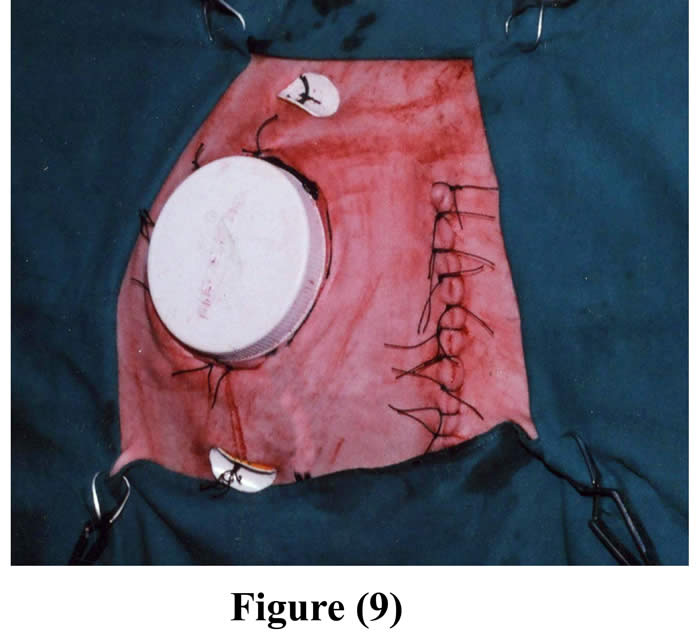
The initial incision through the skin, muscle and peritoneum was sutured as usual. 2-4 pairs of fixation buttons were implanted inside the cannulated rumen and outside the body, through the outer opening of the cannula, around the contour of the cannula, by using silk, and then they were perfectly tighten and tied (Figs 8 & 9). Following operation, animals were isolated, fasted for another 12 hours, administered intravenous fluid therapy, intramuscular long acting oxytetracycline, the wounds were dressed with povidone iodine (Fig 10), food intake was restricted up to one week post surgery to avoid complications, and finally the skin sutures were removed 10 days post surgery.

Results
Fastening of the animals prior to surgery reduced ruminal contents and facilitated surgical interference. Complete muscular relaxation could be achieved after intravenous injection of diazepam and thiopental sodium, and the duration of anesthesia lasted about 90 minutes, followed by smooth uncomplicated recovery. Light polypropylene flexible cannulas (28-35 gm) facilitated its insertion, exteriorization, and fixation and caused no mechanical pain for the operated animals. The average time of the operation from the initial skin incision till the final exteriorization and fixation of the cannula was 55 minutes (50-60 minutes). Cannulas were successfully implanted within the ruminal fistula of 11 ram (5 animals by small cannulas, and 6 animals by large cannulas). The operated animals followed up for 60 days (Figs 11 & 12).
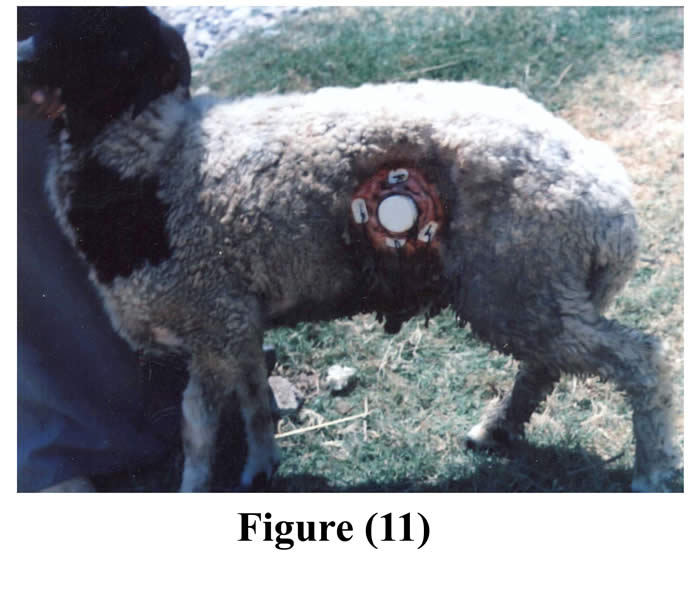
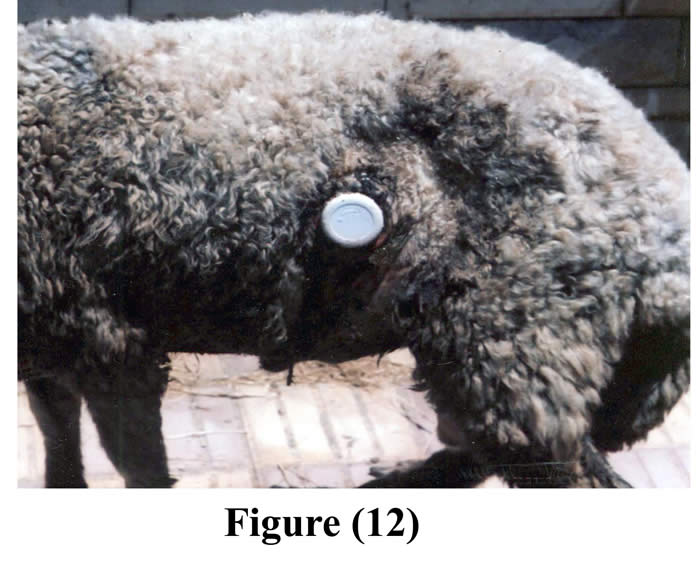
The initial surgical wound showed healing with first intension within 10 days without post surgical problems in any of the operated animals, except presence of temporary slight edema and thickening around the cannula in three animals, and death of one animal due to causes unrelated to surgery, rest of the animals enjoyed good health through the period of experiment (16 months). The cannula was kept in place without any problems, caused no complications or leakage around it; and it remained gas and liquid tight over the period of experiment.
Discussion
Our observations in this study indicated that excessive preoperative preparation reduced the size of the rumen too much to be manipulated and facilitated aseptic surgery. This coincides with Wakanker et al, (1980) who stated that fastening prior to surgery reduced the load of rumen that facilitated healing of the organ and reduced chance of soiling of the surgical field with ruminal contents during surgery. Intravenous injection of diazepam that was followed by thiopental sodium ensured sedation, analgesia, complete muscular relaxation, and control of visceral reflex to efficient and satisfactory levels to perform laparotomy (Tantawy, 1978 and Ragab, 1989). Exteriorization of the cannula through a second incision, which fitted exactly to its diameter, favored healing of the initial incision by first intension and prevented leakage between the rumen and abdominal wall (El-Monzaly, 1975). Exteriorization of the cannulated part of the rumen from peritoneal cavity via a second circular abdominal wound and suturing of the prolapsed part of the rumen, peritoneum, and abdominal muscles around the contour of the cannula to induce incarcerated hernia, ensured mechanical support to the cannula (Venugopalan, 1986). The purse string suture effectively sealed the peritoneal cavity around the level of the cannula as evidenced by absence of peritonitis, on the other hand, Wakanker et al, (1980) mentioned that the through-and-through sutures increased the area of adhesion as expected and prolonged the operative procedure. Slightly smaller incisions of the rumen and skin than the size of neck of the cannula are preferred, that made the rumuinal opening and exposure skin opening fit tightly around the neck of the cannula (Schnautz, 1957 and El-Monzaly, 1975). A purse string sutures around the neck of the cannula tends to curl the edges of the rumen and the skin inward ensuring that the ruminal serosa will come in contact with the cut edges of the skin and subcutaneous layer to provide perfect adhesion and to strangulate the neck of the cannula to support it (Komarek et al, 1961). Placing of 2-4 fixation buttons inside the concha of the cannula and fixation of them by silk to another four buttons over the skin provided tight contact between cannulated part of rumen and abdominal wall as well as ensured mechanical support to the cannula (Misra et al, 1981 and Buckner, 1995). Light weight of the cannula as well as short protruded part of it (1-1.5 cm neck length) through the flank, rarely subjected it to mechanical disturbances (Komarek et al, 1961).
Conclusion
This fabricated cannula is cheap, applicable, durable, and available; causes no mechanical pain; and maintains normal ruminal environment, and this technique of ruminal fistulation and cannulation is simple, easy, with minimal time consuming (one-stage operation), suitable for collection of all ruminal contents, and has no adverse effect on general health condition, so it is advised to be adopted in small ruminants.
References
Abdel-fattah, M., 1999. The goat as a model for experimental surgery. Ph. D. thesis, surgery, anesthesiology, and radiology, Fac. Vet. Med., Cairo Univ., Beni-Suef brnch, Egypt.
Anderson, J. F., Frederikson, E. D., 1976. Surgical fistula as an aid in the treatment of chronic bloat in cattle. Vet. Med. Small Anim. Clin. 71, 1363-1367.
Brown, G. F., Armstong, D. G., MacRac, J. C., 1968. The establishment in one operation of cannula into the rumen and re-entrant cannula into the duodenum and ileum of the sheep. British Vet. J. 124, 78-81.
Buckner, R.,1995. Surgical correction of left displaced abomasums in cattle. Vet. Record 136, 265-267.
Corley, R. N., Murphy, M. R., Lucena, J., Panno, S.V., 1999. Technical note: A device for obtaining Time integrated samples of ruminal fluids. J. Anim. Sci. 77, 2540-2544.
Dougherty, R. W., 1981. Experimental surgery in farm animals. 1st ed . Ames, Iowa , The Iowa state university press.
El-Monzaly, M., 1975. Simplified surgery for rumen cannulation of goats under the influence of Rumpun. J. Egypt. Vet. Med. Assoc.35, 207-218.
Hassanein, A., Soliman, A. S., Eifat, J. F., 1988. Rumen cannulation in sheep. Alexanderia. J. Vet. Sci. 4, 429-435.
Hecker, J. F., 1974. Experimental surgery on small ruminants. Butterworths & Co, Ltd, London, England.
Komarek, R. J., Leffel, E. C., 1961. Gas-tight cannula for rumen fistula. J. Anim. Sci. 20, 982-984.
Misra, S. S., Angelo, S. J., 1981.Vaginopexy technique for the management of recurrent utero-vaginal prolapse in bovines. Ind. Vet. J. 58, 576-580.
Nangeroni, L. L., 1954. A steer with a twelve-year old rumen fistula. J. A. V. M. A. 125, 451-452.
Nocek, J. E., Allman, J. G., Kautzw, P., 2002. Evaluation of an indwelling ruminal probe methodology and effect of grain level on diurnal pH variation in dairy cattle. J. Dairy Sci. 85, 422-428.
Ragab, G. A., 1989. Some studies on the surgery of the urogenital system in small ruminants. Ph. D. thesis, Fac. Vet. Med., Cairo Univ., Egypt.
Santra, A., Karim, S. A., 2002.Rumen cannulation in sheep and goats: Fabrication of cannula and surgical procedure for its implantation. Ind. J. Anim. Sci. 72, 978-980.
Schnautz, J. O., 1957. A rumen fistula modification. Am. J. Vet. Res.18, 73-75.
Tantawy, M., 1978. Studies on the effect of some anesthetics in sheep and goats. Ph. D. thesis Vet. Surg., Assuit Univ., Egypt.
Thyfaultt, H. A., Leffel, E. C., Huang, M. D., 1975. Simplified method for producing permanent rumen fistula. J. Dairy Science 58, 1899-1901.
Venugopalan, A., 1986. Essentials of veterinary surgery. 5th ed., Oxford and IBH publishing Co. PVT. L.T.D.; New Delhi-Bombay.
Wakanker, C. C., Mantri, M. B., Deshpande, K. S., 1980. A study on evaluation of rumen fistulation techniques in bovines. Indian Vet. J. 57, 160-163.
Wayne, B., Lynn, F. J., 1959. A plastic rumen fistula apparatus for sheep – its insertion and use. J. A. V. M. A. 15, 603-605.
Figure1: Fabricated small cannula
Figure 2: Fabricated large cannula
Figure 3: The cannula with a gas collecting tube
Figure 4: Final shape of the cannula
Figure 5: Seat of initial and second incisions on the left flank
Figure 6: The cannula fixed into the rumen through initial incision
Figure 7: The cannula fixed into the rumen through initial incision with the ruminal mucosa shows after removal of the cover
Figure 8: The cannula in position through the second incision with fixation by buttons
Figure 9: Final shape of the cannula in position
Figure 10: The cannula during the 1st week
Figure 11 & 12: The cannula in position after 2 months
First Published December 2007
Copyright Priory Lodge Education Limited 2007
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


