A. Lupi°, G. Gambarini§, G. Bolognini§, L.Testarelli §i,
C.Chimenti^, M.Cordaro *, M. Castagnola*, L. Ceccarelli*, G. Nocca*, R. Scatena*,
and B. Giardina*.
°CNR Centro Chimica dei Recettori c/o U.C.S.C., § Dental Institute
University of Rome “La Sapienza”,. *Dental Schhol & Institute
of Biochemical and Clinical Biochemical, U.C.S.C., Rome. ^ University of L’Aquila
Dental School,
Summary
The purpose of the study was to evaluate cytotoxicity of five commercially available
dental adhesives. Extracts of the tested materials were taken in cell culture
medium of freshly-cured and aged sample. Mouse 3T3 fibroblasts were exposed
to the extracts for 24 hours and the cytotoxicity was evaluated using neutral
red uptake (NRU) assay. Direct toxicity was evaluated also with NRU test.
Results showed that mean values of citotoxicity of freshly cured adhesives were
50%; only one adhesive showed higher values (78%) of toxicity.Aging the samples
affected the cytotoxicity of the extracts to a varying degree. Two products
showed reduced cytotoxicity in NRU, while three adhesives didn’t show
any difference in cytotoxicity respect to freshly extracts.
All the dentin bonding materials were cytotoxic when tested in direct toxicity
in NRU. (about 80%). Only one adhesive showed a lower degree (%) of toxicity.
In conclusion, NRU test permitted to rapidly evaluate cytotoxicity of different
dental materials, and in same time, to address further biochemical and toxicological
studies to identify the molecular mechanisms involved in this toxicity.
Introduction
Dental adhesive systems were introduced in the middle 1960s. The original bonding
formulations essentially were chemically identical to the composite resin but
contained diluents for more wetting the cavity wall (1). The modern adhesive
systems are fourth and fifth generation dentin bonding agents. Generation means
of differentiating between various levels of improved handling characteristics
and clinical performance. In general sense, the fourth generation dentin adhesives
require the use of an adhesive component in addition to the primer to affect
bonding of the overlying composite to the prepared dentinal surface. The fifth
generation dentin bonding agents permit bonding of the composite restoration
without the aid of the traditional adhesive component. Apparently, the wettability
of the primer has been so improved that an adhesive agent is not required to
effect proper wetting by the restorative agent.
The objective of in vitro biocompatibility tests is to simulate biological reactions
to materials when they are placed on or into tissue of the body. Cell culture
systems provide convenient, controllable and repeatable means for an initial
assessment of biological responses (2).There are a number of cytotoxicity tests
available measuring a variety of parameters. The toxicity parameters should
ideally be appropriate to the chemical nature of the test material (3). Lipophilic
substances are more likely to disrupt membrane integrity, therefore a permeability
assay such as neutral red uptake (NRU) is more useful. The NRU assay is so-called
vital staining procedure (4; 5). Viable cells incubated in the presence of NR
take up and retain the dye compound. On the exposure to substances damaging
the plasma or lysosomal membranes, the cell no longer retains NR. Determination
of the amount of retained NR in cells exposed to test compounds, comparated
with controls, enables the relative toxicity of test chemicals to be assessed.
Nru has been applied to ranking cytotoxicity of chemicals according to potency,
and to elucidate their structure-toxicity relationships (6). In this study,
NRU test has been employed to assay the cytotoxic effects of five adhesives
on mouse 3T3 fibroblast cell line, in order to elucidate the cytotoxic potential
of these materials.
Clinically an accurate evaluation of cytotoxic properties of different dental
materials in general and of adhesives in particular could help to reduce the
incidence of early and late complications of different orthodontic implants,
moreover could preserve patients from effects related to systemic toxicity.
In general more attention shouold be paid to tolerability and biocompatibility
data of dental material.Ameliorating the oral pathophysiology related to dental
remodelling could yield positive consequences in clinical and economic terms.
Materials and methods
Materials and reagents. Unless indicated all chemicals and reagents
(cell culture grade) were obtained from Sigma Chemical Co., Milan, Italy.
Cells and Treatments. Mouse 3T3-fibroblasts (swiss albino mouse cell
line) (Istituto Zooprofilattico, brescia, Italy) were grown in a 5% CO2 atmosphere
at 37°C in DMEM (Dulbecco Modified Eagle Medium), 10 mM Hepes, Glucose (1
g/L), NaHCO3 (3,7 g/L), penicillin (100 units/mL) streptomycin (100 mg/mL) and
10% FCS (Fetal Calf Serum) were utilized.
In order to evaluate the cytotoxic effects of the chemicals released in DMEM
from the dentin bonding agents of interest to the present study, 1 x 104 fibroblast
in 200 mL DMEM were seeded per individual well of 96-well tissue culture plate
(Costar, Cambridge, MA), and cultured to subconfluent monolayer for 48 hours.
Then, the DMEM–extracts of the adhesives were added to monolayers by medium
change. Similar volumes of DMEM without extracted substances were added to control
wells. After 24 h of incubation cellular vitality was evaluated by neutral red
uptake (NRU) which is a measure of membrane permeability.
Detailed procedures for
these measurements have been described by Borenfreund and Puerner (4). In brief:
50 mg/mL of neutral red was add to each well. After incubation for 4 h at 37°
C, the supernatant was discarded and the intracellular neutral red were solubilized
with 200 ml of 1% acetic acid in ethanol 50%. The absorbance of each 96-well
plate was determined using an automatic microplate photometer (Packard SpectracountTM,
Packard BioScience Company , Meriden U.S.A.) at 540 nm.
The cell cytotoxicity was calculated according to the equation (7):
% cell mortality
= Control OD – sample OD x 100
Control OD
Each experiment was perfumed in sestuplicate.
Extracts toxicity studies.
Adhesives extract preparation. In order to evaluate the cytopathic
effects of five photopolymerized adhesive substances, thirty-five round filter
papers (whatman, U.S.A.), 7 mm in diameter, were kept in 70% ethanol for 6 h.
After rinsing the papers in PBS, they were dried for 4 hours. On the filter
papers, 5 mL of each dentin adhesive materials was applied and light-cured for
20 s (BLUELIGHT pro Mectron medical technology), light source at a standardised
distance of 5 mm
Six round filter papers were used for each experimental material (Table
1)
After polymerisation, the adhesive materials were plated on 24 wells plates
in for 24 h or for six days (surface to liquid ratio: 11.8 mm2/mL).
Direct toxicity
In order to evaluate the direct cytopathic effects of the photopolymerized adhesive
materials, cells of 3T3 cell line were plated at 30.000 cells/cm2 on bottom
of every well of two 24-wells plates and maintained for 72 h. Then the round
filter papers with and without (control) adhesives were added to monolayer.
After 24 h of incubation the cell viability was evaluated by NRU test.
Statistical Analysis
Each value represents the mean of three experiments, using six replicates of
each material per experiment.
All results are expressed as mean SEM. The group means were compared by analysis
of variance (ANOVA) followed by a multiple comparison of means by Student-Newman-Keuls.
Upon occurence comparison of means by T-Student test was used.
p < 0.05 was considered significant.
Results
24 hours-extract toxicity:
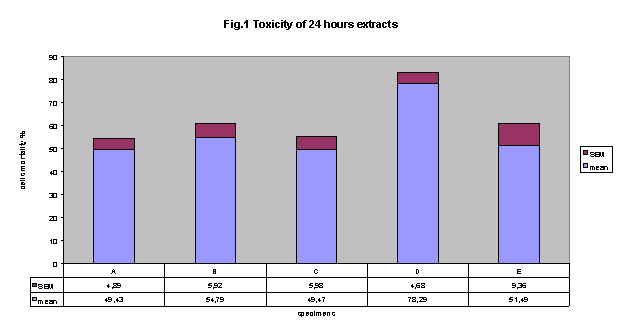
Figure 1 shows cytotoxicity data regarding extracts of freshly cured of five
commercially available adhesives, using NRU test. In this experimental conditions,
results clearly indicate that all adhesives showed an important cytotoxicity
with respect to control. Importantly, extract by adhesive D showed the higher
incidence of cytotoxicity (78.3% ± 4.7 SE). However, this cytotoxicity
resulted statistically significant different only versus adhesives A and C (cytotoxicity:
49.4% ± 4.9 and 49.5 ± 6.0, respectively).
Six days-extracts toxicity:
Medium conditioned for six days with the five adhesives showed interesting data
in term of toxicity using NRU test.
Fig. 2 shows that all adhesive extracts were cytotoxic with respect to control.
Interestingly, cytotoxicity comparison between six days-extracts and 24 hours-extracts
permit to realise some considerations:
A, B and E did not show any difference in cytotoxicity with respect to time
of incubation of adhesive.
Adhesive D showed a significant reduction in cytotoxicity related to time (%
cell mortality 24 hs: 78.3 ± 4.68 versus six days: 52.6 ± 10.64;
p < 0.05);
Adhesive C showed a significant reduction in cytotoxicity related to time (%
cell mortality: 24 hs: 49.5 ± 5.98 versus 6 days: 15.3 ± 4.63;
p < 0.01);
In conclusion, these data seem to show that membrane damage, induced by adhesive
C to 3T3 mouse fibroblast cell line in this experimental condition, is less
than all other adhesive. However, this difference in cytotoxicity was statistically
significant (p <0.05) only with respect to adhesive D.
Direct toxicity:
All of the dentin bonding materials were cytotoxic when tested in direct toxicity
in NRU (fig.3) .
A 84.6%, B 76%, C 83%, D 82% and E 53% .
Statistically significant difference was found between the cytotoxicity of E
adhesive and all other materials. (E versus A p< 0.01, E versus B p< 0.01,
E versus C p< 0.01 and E versus D p< 0.001,).
Interestingly, direct toxicity induced by adhesives A, B and C was statistically
higher than one’s by freshly extracts (p< 0.0001, p<0.0001, p<0.05,
respectively). Intriguingly, comparison between direct and extract toxicity
of adhesives D and E did not show any significant difference.
Discussion
Biocompatibility may be defined as the ability of a material to function in
a specific application in the presence of an appropriate host response (8).
The need for biocompatible materials implies the necessity of toxicity testing.
The toxicity of a dental material can be evaluated by in vitro tests, and by
clinical studies in humans. In vitro studies are mainly perfomed to evaluate
the cytotoxicity (cell damage) or the genotoxicity (specific DNA damage or chromosomal
aberration) of a dental material).
In the present study the biological compatibility of five dental adhesives were
examined by in vitro methods. NRU test is considered a fast, valid and reproducible
method to evaluate cytotoxicity in vitro, in particular it seems to stress damage
to biological membranes in general and plasmatic and lysosomal membranes in
particular. In this study, as determined by NRU test adhesives are cytotoxic
following application on 3T3 mouse fibroblast cell line. The statistical analysis
demonstrated that all extracts reduced cellular viability about 50%, only adhesive
Scotchbond 1 ( adhesive D) showed a cytotoxic activity of about 80% (see table
2). Considering six-day extract cytotoxicity, only two samples One Q Bond
and Scotchbond 1 (adhesive C e D), showed a decrease in cytotoxicity with respect
to corresponding 24 hours-extracts. Interestingly, toxicity induced by Solist,
OptiBond Solo Plus and Excite (adhesive A B and E, respectively) did not show
any difference respect to 24 hours-extracts.
In direct toxicity all adhesives reduced cellular viability about 80%, only
Excite (adhesive E) showed a cytotoxic activity about 50% .
In conclusion, NRU test permitted to rapidly evaluate cytotoxicity of different
dental material, and in same time, to address further biochemical and toxicological
studies to identify the molecular mechanisms involved in this toxicity.
In particular, comparative studies with others cytotoxicity tests (like the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) test) are
in program for better clarify the mechanisms of cytopathic effects of dental
adhesives.
References
1. Leinfelder K.F. Current status of dentin adhesive systems. Alpha Omegan,
91, 4, 1998.
2. Scuster GS, Lefebvre CA, Wataha JC, White SN. Biocompatibility of posterior
restorative materials. J Calif Dent Assoc. 1996; 24: 17-31.
3. Schweikl H, Schmalz G. Toxicity parameters for cytotoxicity testing of dental
materials in two different mammalian cell lines. Eur J Oral Sci 1996; 104:292-9.
4. Borenfreund E, Puerner JA. A simple quantitative procedure using monolayer
cultures for Cytotoxicity assay (HTD/NR-90) J Tiss Cult Meth 1984; 9:7-9.
5. Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological
alterations and neutral red absorption. Toxicology Lett 1985; 24: 119-24.
6. Babich H, Borenfreund E Applications of the neutral red Cytotoxicity assay
to in vitro toxicology. ATLA 1990; 18: 129-44.
7. Hashieh IA, Cosset A. Franquin JC, Camps J In vitro cytotoxicity of one-step
dentin bonding systems. Journal of endodontics 25, 2. February 1999.
8. Schmalz G. Use of cell cultures for toxicity testing of dental materials-advantages
and limitations. J. Dent. Suppl. 2, 1994; 22: S6-S11.
Table 1: Characteristics of adhesives.
| Adhesive | Lot No | Manufacturer | Marked |
| Slist | 00480030 | DMG Germany | A |
| Optibond | 103517 | KERR Corporation USA | B |
| ONE Q Bond | 06908011 | Colloidal Glass Technology | C |
| Scotchbond 1 | 4242 | 3M Dental Products | D |
| Excite | B29610 | Vivadent Lichtenstein | E |
Table 2: Cytotoxicity comparison between six days-extracts
and 24 hours-extracts
| Adhesive materials | Cell mortality’ % ± SEM (freshly extracts) | Cell mortality’ % ± SEM (aged extracts) | p (t-test) |
| A | 49.43 ± 4.89 | 36.09 ± 7.37 | >0.05 |
| B | 54.79 ± 5.92 | 44.1 ± 10.99 | >0.05 |
| C | 49.47 ± 5.98 | 15.33 ± 4.63 | <0.001 |
| D | 78.29 ± 4.68 | 52.59 ± 10.64 | <0.05 |
| E | 51.49 ± 9.36 | 46.69 ± 11.08 | >0.05 |
A, B and E did not show any difference in cytotoxicity with respect to time
of incubation of adhesive. Adhesive D showed a significant reduction in cytotoxicity
related to time (% cell mortality 24 hs: 78.3 ± 4.68 versus six days:
52.6 ± 10.64; p < 0.05); adhesive C showed a significant reduction
in cytotoxicity related to time (% cell mortality: 24 hs: 49.5 ± 5.98
versus 6 days: 15.3 ± 4.63; p < 0.01).
Figures

Figure 1 cytotoxicity of
24 hours-extracts: All adhesives were cytotoxic. Extract by adhesive D showed
the higher incidence of cytotoxicity (78.3%±4.7 SEM). this cytotoxicity
resulted statistically significant versus adhesives A and C (cytotoxicity: 49.4%
± 4.9 and 49.5 ± 6.0, respectively, p< 0.01).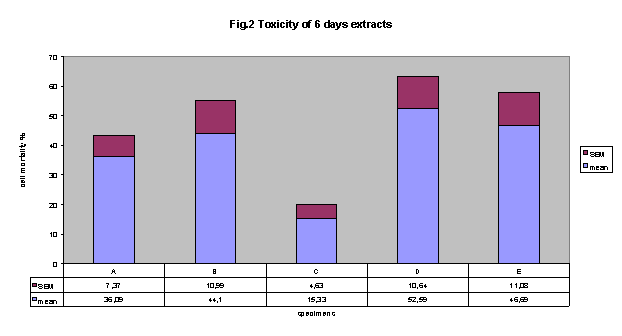
Figure 2 cytotoxicity of 6 days-extracts: All adhesives were cytotoxic. adhesive
C is less toxic than all other adhesive. This difference in cytotoxicity was
statistically significant (p <0.05) only with respect to adhesive D.
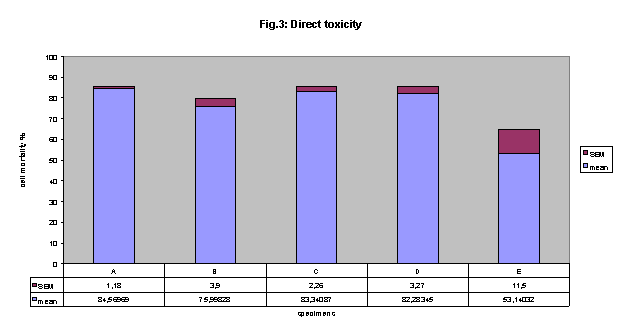
Figure 3 direct cytotoxicity: All of the dentin bonding materials were cytotoxic.
Statistically significant difference was found between the cytotoxicity of E
adhesive and all other materials. (E versus A p< 0.01, E versus B p< 0.01,
E versus C p< 0.01 and E versus D p< 0.001,).