Browse through our Journals...
Infective Endocarditis and Antibiotic Prophylaxis: A Systematic Review of Efficacy, and Safety of the AHA Guidelines
Michael W. Tempelhof, MD, Gordon Reeves, MD,
From the Department of Cardiovascular Medicine, Division of Internal Medicine, Northwestern University Medical Center. Chicago, IL.
Address for correspondence: Michael W. Tempelhof, MD, Northwestern University Medical Center, 251 Huron St Chicago, IL 60611. Phone: 919-824-1180.
ABSTRACT
Background This study examined the efficacy, safety, and cost-effectiveness of administering antibiotic prophylaxis prior to endodontic procedure in an effort to clarify the 2007 IE prevention guidelines.
Methods A nonparametric, meta-analysis of studies reporting antibiotic efficacy was executed. Antibiotic safety analysis was reported as IE cases prevented compared with antibiotic-associated deaths per 10 million patients receiving prophylaxis. Cost-effective analysis was reported in Quality Adjusted Life Years (QALY).
Results No data exists demonstrating that a decreased frequency of bacteremias confers an IE prevention benefit. The pooled adjusted odds ratio (OR) for the development of IE with antibiotic prophylaxis among the four case-control studies was highly heterogenous and statistically non-significant (0.48 [95% CI (0.2-1.16) p value = 0.10]). Chemoprophylaxis utilizing amoxicillin or ampicillin presents a higher risk of fatal adverse drug reactions (20 cases per 1 million patients treated) then cephalosporin, macrolide and clindamycin regimens (0.5-5.7 cases per 10 million patients treated). IE chemoprophylaxis to moderate-risk patients costs, on average $96,174 per QALY saved, exceeding the cost-effectiveness threshold. Oral chemoprophylactic therapy to high-risk patients is a cost-effective practice with an average cost of $29,290 per QALY.
Conclusions The AHA, 2007 IE prevention guidelines appropriately reflect the efficacy, safety and cost-effective evidence for IE prophylaxis. Antibiotic administration to moderate and high-risk patients prior to endodontic procedure provides minimal to no protective efficacy. The administration of oral chemoprophylaxis prior to endodontic procedure only to patients with a high-risk of adverse outcomes subsequent to the acquisition of IE is a beneficial, safe and cost-effective practice.
KEY WORDS : Infective Endocarditis Antibiotic prophylaxis, prevention guidelines,
Infective Endocarditis (IE) is an uncommon condition with an incidence rate of 3.95 cases per 100,000 person-years yet, has an overall high mortality rate of 20-26% (Mylonakis E,et al 2001). Despite the implementation of antibiotics into prophylactic practice during the 1940’s and recent advances in the medical and surgical management of IE, the overall incidence and outcomes of IE remain unchanged. (Mylonakis E,et al 2001, Weinstein L et al 1996, Di Filippo S et al, 2006).
Since the 1920’s, endodontic procedures have been associated with a high incidence of bacteremia; therefore, dental procedures were implicated as an independent risk factor for the development of bacterial endocarditis (Bender I et al 1960, Rabinovich S et al, 1965). IE prevention practices advocated for the administration of pre-endodontic procedural antibiotics to reduce the risks associated with endodontic-induced bacteremias. In 1955, the American Heart Association (AHA) published the first of ten subsequent IE prevention guidelines. The 2007, AHA IE prevention guidelines underwent changes intended to clarify patient eligibility criteria for receiving IE prophylaxis (Table 1) (Wilson et al, 2007). Major changes include, the consideration that frequent exposures to bacteremias associated with daily activities are considered more likely to induce IE then are endodontic-procedural induced bacteremias. Optimal oral hygiene is emphasized as an important practice for IE prevention. A patient’s lifetime acquisition risk of IE is no longer a consideration for initiating prophylactic antibiotic therapy. The AHA now recommends the administration of single-dose prophylactic antibiotics prior to endodontic procedure only to patients with cardiac conditions associated with the highest risk of adverse outcomes following the acquisition of bacterial endocarditis (Table 2) (Wilson et al, 2007). The new practice patterns associated with the 2007 AHA guidelines have raised concerns among dentists, physicians and patients. In concordance with the new recommendations, healthcare providers withhold prophylactic therapy to a substantial number of patients who previously received pre-procedural antibiotic prophylaxis. Healthcare providers who practice according to the new IE prevention guidelines are frequently met with inquiry and objection from both colleague and patients’.
In this systematic review, we report the results of a comprehensive literature review and meta-analysis assessing the evidence for providing pre-endodontic procedural antibiotic prophylaxis as an IE prevention practice according to the 2007, AHA IE prevention guidelines. Evidence addressing the efficacy and safety of antibiotic administration prior to endodontic procedure for IE prophylaxis were of particular interest.
METHODS
Literature review and abstraction.
A comprehensive search of the literature was performed. The search strategy was developed on MEDLINE PubMed, Cochranee Controlled Trials Register (CENTRAL), OLDMEDLINE (Ovid, 1966 to June 2002), EMBASE (Ovid, 1980 to June 2002) and International Pharmaceutical Abstracts (IPA) for relevant articles published in English with no publication date limits. The medical subject headings and text words used were infective endocarditis, antibiotic, antibiotic prophylaxis, chemoprophylaxis, bacteremia, endodontic procedures, dentistry, safety, quality adjusted life years and efficacy. No limits on combinations of inclusion terms were used. References of reviewed articles were also searched for relevant articles.
One reviewer independently conducted the literature search and abstraction of relevant articles. The inclusion criteria included randomized controlled trials, cohort studies, case-control studies and cross-sectional studies in humans, animals and in vitro models. Data and quality information were abstracted onto a custom data collection form. Abstracted characteristics of studies included author, year, study design, country of origin, duration, details of antibiotic intervention, types of dental procedure, underlying cardiac condition, matched population variables and population IE-risk factors. Outcomes data collected were number of deaths, new cases of endocarditis, cases of IE prevented, population randomization, quality-adjusted life year (QALY) per intervention, and cost-effectiveness comparison per intervention.
Statistical Analysis
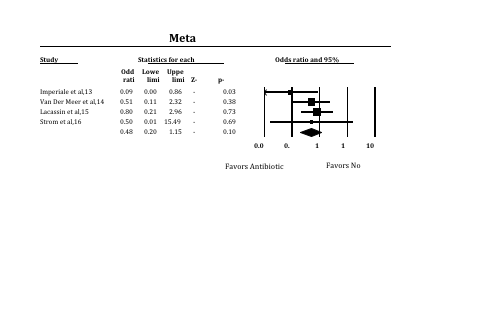
Clinical studies which analyzed the protective efficacy of administering pre-endodontic antibiotics for IE prevention were assessed for conceptual and statistical heterogeneity. Heterogeneity between trial results was tested using a standard chi squared test, and considered significant where p < 0.1. FAST*PRO software was used to calculate the protective efficacy of antibiotic prophylaxis with associated confidence intervals based on the reported findings for each study (figure 1). A forest plot (figure 1) and chi-square analysis were completed to asses for conceptual and statistical heterogeneity respectively.
RESULTS
State of the Evidence. No randomized controlled trials assessing whether the administration of antibiotics to at-risk patients prior to bacteremia-inducing endodontic procedures confers a protective benefit against the development of IE have been completed. Previous AHA recommendations were extrapolated from in vitro susceptibility data of pathogens inducing endocarditis, prophylactic studies in experimental animal models, pharmacokinetic studies, and observational analyses of endocarditis in humans (Girard A et al, 1993, Rouse M S et al, 1997 ).
Numerous pre-clinical studies, expert reviews and editorials have questioned the appropriateness of previous AHA IE prevention recommendations (Bashore TM et al 2006, Seymour R A et al, 2000, Agha Z et al, 2005).
The dogma that high-risk dental procedures produce a bacteremia of significant magnitude to induce IE has been questioned. Four case-control studies and one extrapolated population report calculated the IE acquisition risk in subjects receiving and not receiving antibiotic prophylaxis prior to endodontic procedure (Imperiale TF et al, 1990, Van Der Meer JT et al, 1992, Lacassin F et al, 1995, Strom B et al, 1998, Duval X et al, 2006). Recent data have questioned the safety of antibiotic administration to such large populations (Agha Z et al, 2005).
Pathophysiology
The pathophysiology of new onset IE remains un-elucidated. There is a prerequisite for the existence of a bacteremia of significant intensity. One currently accepted theory suggests that conditions associated with mechanical destruction of the endocardium exposes the subendothelium; thus, promoting local activation of the clotting cascade. Microbial pathogens (Streptococcus viridans representing 50% of the reported cases) associated with transient bacteremias from dental procedures, routine daily activities and otherwise bind to and activate inflammatory cytokines on the coagulum resulting in progressive enlargement of an infected vegetation (Heimdahl A et al 1990). A second theory describes other bacteria, most notably Staphylococcus aureus, which express surface fibronectin. Fibronectin facilitates bacterial adherence to the locally inflamed endothelium. The interaction of bacteria and inflammation initiates the milieu of a progressive vegetation (Prendergast BD et al, 2006). Subsequent local extension and tissue damage results in septic emboli disseminating to the brain, spleen, kidney and peripheral vasculature, contributing to the sequelae of IE first described by Osler.
Endodontic Procedures, Bacteremia and Infective Endocarditis Risk.
Eighty to ninety percent of high-risk endodontic procedures induce a transient bacteremia in humans (Roberts G et al, 1994, Lee CE et al, 2000). However, only 4%-7.5% of all bacterial endocarditis cases are related to endodontic associated bacteremia (Gendron R et al, 2000). Following dental intervention, the absolute risk for developing IE is estimated at 1 case per 14 million dental procedures, with the IE acquisition risk for high-risk patients with underlying cardiac conditions following dental intervention being substantially higher (Pallasch TJ et al, 2003). IE acquisition risk estimates following an endodontic induced bacteremia in patients with congenital heart disease are 1 per 475,000; rheumatic heart disease, 1 per 142,000; prosthetic heart valve, 1 per 114,000; and previous IE, 1 per 95,000 dental procedures Pallasch TJ et al, 2003).
The correlation of bacteremia frequency and duration on the acquisition risk of IE has not been assessed. However, considering the average American undergoes 2 dental visits per year (which may or may not include endodontic procedure), the frequency of exposure to an endodontic induced bacteremia is minimal. Transient bacteremia associated with endodontic procedure are of relative short duration. Blood cultures in humans remain positive for only 30 to 60 minutes immediately following endodontic procedure. The importance of bacterial inoculum magnitude has been demonstrated by experimental catheter-induced valvular insult in rabbit models. These studies established that inocula of 1 x 108 (100 million) colony forming units [cfu]/mL or greater are required to consistently induce experimental endocarditis (Carrion PK et al , 1970, Bahn SL et al, 1978, Crémieux AC et al, 1993). Recent human quantitative blood culture data support the implication that endodontic associated bacteremia inocula are of insufficient magnitude to induce endocarditis. Bacteremia intensities immediately following invasive human dental procedures peaked at 1.5 cfu/ml-5.9 cfu/ml and quickly precipitated over the proceeding minutes to hours (Roberts GJ, 1993). In retrospect, these are relatively low inocula; 5-6 orders of magnitude lower then the experimental model inocula intensities suggested were necessary to induce endocarditis.
Ironically, in their 1942 paper advocating antibiotic prophylaxis, Northrop et al, 1943, discovered that only 20% of confirmed cases of IE were associated with an endodontic procedure in the preceding 3 months. Evidence which suggested that new onset IE may not be attributed to endodontic procedure. Recent reports have confirmed low rates of previous dental intervention in association with new onset IE. In all, five reports have demonstrated that 0-17% of patients diagnosed with IE had undergone a dental procedure 30-180 days prior to diagnosis (Imperiale TF et al, 1990, Van Der Meer JT et al, 1992, Lacassin F et al, 1995, Strom B et al, 1998, Duval X et al, 2006). The most recent case-control study of 104 patients with known, high-risk structural heart disease discovered that patients who developed IE were actually less likely to have experienced an endodontic procedure within the 180 days prior to diagnosis then did control patients who did not develop IE (OR 0.2 [95% CI 0.04-0.7]) (Strom B et al, 1998). Risk for new onset IE and experiencing an endodontic procedure in the preceding 90 days was not demonstrated, (OR 1.2 [95% CI 0.7-2.1]). A follow-up analysis confirmed these early results, concluding that IE was not associated with endodontic or other previously defined high-risk procedures. Among high-risk patients with underlying structural heart disease, kidney disease (OR 16.9 [95% CI 1.5-193.0]), diabetes (OR 2.7 [95% CI 1.4-5.2]) and skin flora infection (OR 3.5 [95% CI 0.7-17.0]) were associated with a greater risk for the development of bacterial endocarditis (Strom BL et al, 2000). The role endodontic procedure induced bacteremia has on inducing IE remains inconclusive. Investigators have therefore assessed the IE acquisition risk associated with other known causes of transient bacteremias.
Daily activities such as chewing and oral hygiene practices result in bacteremias more frequently, of longer duration and of greater magnitude in comparison to high-risk endodontic procedures (Table 3) (Seymour R A et al, 2000, Roberts GJ, 1999). However, the duration and magnitude of bacterial inocula associated with routine activities are still remarkably low and may not be a risk factor for the induction of bacterial endocarditis (Seymour R A et al, 2000, Roberts GJ, 1999). Evidence supports an emphasis on optimizing oral hygiene to decrease the frequency of bacteremias associated with routine daily activities (Conner HD et al, 1967). Although, no data exists demonstrating that a decreased frequency of bacteremias confers an IE prevention benefit.
These findings have led the AHA to conclude that the cumulative background bacteremia associated with chewing, daily dental hygiene practices, kidney disease, diabetes, and skin colonization present a greater risk of significant bacteremia then any single invasive dental procedure( Wilson et al, 2007). The AHA no longer advocates the lifelong prophylactic administration of antibiotics to at-risk patients to prevent IE from routine daily activities. Optimal oral hygiene is advocated as it may reduce bacteremia frequency associated with daily activities with minimal to no associated risks.
Prophylactic Antibiotic Efficacy
The efficacy of antibiotic regimens for IE prophylaxis has never been assessed under the scrutiny of a randomized controlled trial. Evidence supporting pre-endodontic chemoprophylaxis efficacy is extrapolated from data demonstrating reductions in bacteremia magnitudes immediately following the administration of antibiotics (Lockhart PB, 1996, Lockhart PB et al, 2004). Additionally, recent in-vitro studies suggested that the anti-inflammatory properties of antibiotics reduce bacterial adherence to compromised endocardial tissue and therefore confer an efficacy benefit (Frieling JT et al, 1997). Whether antibiotic prophylaxis is effective for the prevention of endocarditis in patients prior to invasive endodontic procedure remains equivocal.
The Cochrane Collaboration assessed whether prophylactic administration of penicillin to moderate- to high-risk patients prior to endodontic intervention conferred a mortality, serious illness or endocarditis incidence benefit (Oliver R et al, 2004). Their extensive search and exclusion criteria resulted in three case-control studies analyzing patients undergoing any AHA defined moderate- to high-risk oral-dental procedure. Encompassing 350 patients, the 3 case-control trials were published prior to 1997 and reported a range of 46% to 91% decreased incidence of new IE cases among patients receiving pre-endodontic procedural antibiotic therapy versus the control populations’ who did not receive pre-procedural antibiotics (Table 4) (Imperiale TF et al, 1990, Van Der Meer JT et al, 1992, Lacassin F et al, 1995, Strom B et al, 1998). The pooled, adjusted Odds Ratio across all studies for the development of IE among patients receiving prophylaxis was non-significant (0.56 [95% CI (0.15-2.15)]). The Cochranee Collaboration concluded; ‘it is unclear whether antibiotic prophylaxis is effective and there is a lack of evidence to support published guidelines using penicillin as chemoprophylaxis for IE’.
Our systematic literature review identified 4 case-control studies assessing antibiotic efficacy for IE prevention. In addition to the previously mentioned 3 case-control studies reviewed by The Cochranee Collaboration, our review identified an additional case-control study subsequently published in 1998. In a comparison analysis of 29 high-risk patients with known structural heart disease who developed IE within 180 days of experiencing an endodontic procedure with matched, high-risk control patients who did not develop endocarditis, Strom et al,1998 discovered the administration pre-endodontic procedural antibiotics did not provide a protective benefit against the development of IE (OR 0.5 [CI .01-9.6]). Case patients were proportionally more likely to have received AHA guideline appropriate antibiotic prophylaxis then were the risk-equivalent, control patients (Table 4).
The protective efficacy of pre-endodontic antibiotic prophylaxis for IE prevention was calculated for each of the 4 case reports (Figure 1). Interpretation of the results demonstrated a considerable variation in study outcomes. Imperiale et al, 1990 reported a statistically significant protective effect, whereas the remaining studies failed to demonstrate such an effect. Confirmation of statistical heterogeneity was completed first conceptually with a forest plot (Figure 1) and then with chi-square test analysis (chi-square 8.005 for 3 dof, p=0.0459). Large degrees of heterogeneity precluded the completion of a statistically meaningful meta-analysis of the protective effect of antibiotic prophylaxis across all 4 studies.
The most recent report investigating the efficacy of prophylactic antibiotics to high-risk IE patients prior to endodontic procedure was published in 2006 (Table 4) (Duval X et al, 2006). Utilizing a 2805-patient database of patients who developed IE within 3 months of experiencing endodontic intervention, Duval and colleagues estimated the efficacy of providing chemoprophylaxis to all high-risk patients who underwent an endodontic procedure in the preceding 30 days (estimated at 2.7 million). Providing chemoprophylaxis to all 2.7 million high-risk patients predicted a 70% lower incidence of new onset IE, thus preventing 80 new cases of IE. The authors concluded that the risk of adverse drug reactions must be weighed against the high, number needed to treat associated with practicing large population chemoprophylaxis.
Safety Concerns with Antibiotic Prophylaxis.
Adverse reactions associated with the administration of beta-lactam antibiotics are common. Ranging in severity from pruritus to fatal anaphylactic shock, the frequency of all adverse reactions from the administration of penicillin to the general population is 0.7% to 10% (Idsoe O et al, 1968). The incidence of fatal anaphylaxis among patients receiving single-dose penicillin, ampillicin or amoxicillin therapy is approximately 20 cases per 1 million patients treated (Idsoe O et al, 1968, The International Collaborative Study of Severe Anaphylaxis 2003). Single-dose, cephalosporin-associated fatal anaphylaxis risk is estimated at 0.5-5.7 cases per 10 million patients treated (Kelkar P, James T, 2001). Macrolide and clindamycin single-dose fatal anaphylaxis risk is estimated at 0-5 cases per 1 million patients treated (Mazur N et al, 1999). With the highest associated mortality rate of all the recommended antibiotic regimens, the risk of anaphylaxis with the initiation of beta-lactam IE prophylactic therapy must be considered (Lin RY, 1992).
The importance of risk-benefit analysis prior to initiation of IE chemoprophylaxis with beta-lactam therapy was highlighted in a pivotal paper by Bor, et al. Patients with mitral valve prolapse who, according to the then current AHA guidelines appropriately received IE chemoprophylaxis with penicillin were five times more likely to die from an antibiotic induced anaphylactic reaction then from the sequelae of IE (Bor DH et al, 1984). Recommendations for IE prophylaxis with antibiotics to patients with mitral valve prolapse prior to endodontic procedure was rescinded in the proceeding AHA IE prevention guidelines. Subsequent studies have addressed the risk-to-benefit ratio of practicing IE chemoprophylaxis with beta-lactam antibiotics to a broader patient population. The annual risk of mortality associated with the development of endodontic-induced IE is estimated at 26 deaths per 100 million (Bor DH et al, 1984). Whereas the risk of mortality associated with the single-dose administration of beta-lactam antibiotics for IE prophylaxis is estimated at 1-3 anaphylactic deaths per 1 million patients treated (Wilson et al, 2007). The mortality risk associated with single-dose antibiotic therapy has never been assessed under the scrutiny of a prospective trial (Helbling, A et al, 2004).
The 2007 AHA IE prevention guidelines recommend amoxicillin as first-line, IE prevention chemoprophylactic therapy for high-risk patients without a documented type-I hypersensitivity to beta-lactam antibiotics. Amoxicillin provides adequate coverage against oral pathogens commonly implicated in post-dental endocarditis at a fraction of the cost for alternative regimens with comparable in vitro efficacy (Adnan S et al, 1990). Amoxicillin and ampicillin are, as previously stated, associated with a higher risk of fatal anaphylactic reactions then alternative cephalosporin, macrolide and clindamycin regimens. According to the AHA, single dose administration of a beta-lactam antibiotic for IE prophylactic therapy is a safe practice as it has never resulted in a reportable case of fatal anaphylaxis.
DISCUSSION
Infective endocarditis is associated with a high risk of morbidity and mortality. The primary prevention of IE is therefore justified. The presumed correlation of endodontic induced bacteremia, and new onset IE made pre-procedural antibiotic prophylaxis a reasonable practice for the preceding 60 years. However, there is a paucity of evidence in support of providing chemoprophylaxis for effective IE prevention. Bacterial inocula subsequent to endodontic procedures are infrequently experienced, of short duration and of low magnitude. The temporal relationship of endodontic procedure and new onset IE is often prolonged. These findings fail to suggest a causality relationship between endodontic procedures and new onset bacterial endocarditis. No data demonstrates that a decrease in frequency, magnitude or duration of bacteremias from the administration of antibiotics confers an IE prevention benefit. In addition, providing antibiotics for prophylactic practice has associated adverse risks.
Chewing, dental hygiene practices, kidney disease, diabetes, and skin colonization present a greater risk of significant bacteremia and greater cumulative IE acquisition risk then any single invasive dental procedure. Frequency of bacteremias associated with daily activities in at-risk patients are reduced with optimal oral hygiene with minimal to no associated adverse risks. The AHA recommends reducing the incidence of bacteremia with the optimization of oral hygiene in at-risk patients and does not recommend indiscriminant pre-procedural chemoprophylaxis as a safe, IE prevention practice.
The pre-procedural, estimated IE acquisition risk in at-risk patients with underlying cardiac conditions (Table 5) is significantly greater then for negligible-risk patients (Dajani AS et al, 1997). Morbidity and mortality rates associated with high-IE acquisition risk patients are variable and must be considered prior to the initiation of pre-endodontic procedure chemoprophylaxis. Although patients with mitral valve prolapse have a high-IE acquisition risk, the risk of adverse outcomes including death and morbidity is low. As demonstrated by Bor et al, providing antibiotics to this cohort of patients resulted in an excess of treatment associated adverse events compared to the number of adverse outcomes prevented. Other conditions with similar, high-IE acquisition risk and low-adverse outcomes risk following IE acquisition exist. Health care providers must perform a risk-benefit analysis for each individual patient prior to initiation of antibiotic prophylaxis. The 2007 AHA, IE prevention guidelines no longer advocate the administration of pre-endodontic chemoprophylaxis to patients based on their lifetime IE acquisition risk as the risks from providing such therapy may outweigh the benefit. Withholding antibiotic prophylaxis in these circumstances seems a reasonable practice.
No randomized, placebo-controlled trials assessing the protective efficacy of single-dose of antibiotic prophylaxis prior to endodontic procedure for IE prevention have been completed. Case-control studies have reported conflicting results on the protective efficacy conferred from providing pre-procedural antibiotic prophylaxis to at-risk patients. A pooled meta-analysis of the protective benefit from chemoprophylaxis from these case-control studies was not completed as there is a high degree of statistically heterogeneity between the case-control results. Obtaining studies of complete clinical homogeneity was limited by the few published reports addressing IE prevention practices. Potential characteristical differences across reviewed studies include severity differences in predisposing cardiac conditions, inability to rule out confounding IE risk factors and utilization of different antibiotic regimens. Statistical heterogeneity was also attributable to differences in study design and the low incidence of IE (table 4). The AHA acknowledges that even if chemoprophylaxis conferred 100% efficacy, few cases of IE would be prevented as the incidence of endodontic induced IE is so low. Therefore, the goal of prophylactic therapy is to identify patients who would derive the greatest benefit from IE prevention. The AHA now recommends the administration of pre-endodontic procedural prophylactic antibiotics to patients with the highest risk of adverse outcomes subsequent to the development of IE.
The AHA continues to recommend oral amoxicillin as a safe, first-line IE prevention practice for patients without a documented hypersensitivity to beta-lactam antibiotics who are at a high-risk of adverse outcomes from developing IE. Acknowledging an estimated 10-20 fold greater risk of single-dose fatal anaphylaxis with amoxicillin compared to single dose cephalosporin, macrolide and clindamycin regimens, the AHA believes prophylaxis with amoxicillin is a safe practice as there have been no reports of fatal anaphylaxis from a single-dose of pre-dental IE prophylaxis oral amoxicillin. Health care providers must weigh the IE acquisition risk, risk of IE associated adverse outcomes and efficacy benefit from prophylactic antibiotics with the risk of adverse drug reactions for individual patients prior to the administration of antibiotics for IE prevention.
Given the paucity of efficacy data, large estimates of number-needed to treat and adverse risks associated with IE prophylactic practices, the administration of prophylactic antibiotics is a public health policy issue.
Conclusions
Evidence for chemoprophylaxis efficacy remains equivocal and necessitates further investigation. Only a well-designed and adequately-powered randomized controlled trial will provide definitive guidance for the utilization of antibiotic prophylaxis for IE prevention. The current data supports the judicious assessment of a patient’s risk for adverse outcomes subsequent to the acquisition of IE with the potential adverse outcomes associated with pre-endodontic antibiotic prophylaxis. The 2007, AHA IE prevention guidelines advocating chemoprophylaxis for patients with a high-risk of adverse outcomes subsequent to the acquisition of IE appropriately reflects the current data on the efficacy and of antibiotic prophylaxis.
Bacteremia resulting from daily activities is more likely to cause IE than bacteremia associated with a dental procedure.
Even if antibiotic prophylaxis is 100% effective only a small number of cases of IE might be prevented.
Antibiotic prophylaxis is no longer recommended based solely on an increased lifetime risk of acquisition of IE.
Antibiotic prophylaxis prior to dental procedures that involve manipulation of gingival tissues or periapical region of teeth or perforation of oral mucosa is recommended only to patients with underlying cardiac conditions associated with the highest risk of adverse outcome from IE listed in Table 2.
Patients with underlying cardiac conditions associated with the highest risk of adverse outcome from IE as listed in Table 2 are recommended to receive antibiotic prophylaxis prior to procedures on respiratory tract or infected skin, skin structures, or musculoskeletal tissue.
Endocarditis prophylaxis with the administration of pre-procedural antibiotics is not recommended for GU or GI tract procedures, tattooing, ear or body piercing, vaginal delivery and hysterectomy.
|
TABLE 1. Significant Changes in AHA 2007 IE Prevention Guidelines. Adapted from Wilson et al (8).
Prosthetic cardiac valve. Previous IE. Congenital heart disease (CHD): Unrepaired cyanotic CHD, including palliative shunts and conduits. Completely repaired congenital heart defect with prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure. Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization). Cardiac transplantation recipients who develop cardiac valvulopathy. |
TABLE 2. Cardiac Conditions Associated With the Highest Risk of Adverse Outcome from Endocarditis for Which Prophylaxis With Dental Procedures Is Recommended. Reprinted from Wilson et al (8).
Procedure Prevalence of Bacteremia Extractions •single 51% •multiple 68–100% Periodontal surgery •flap procedure 36–88% •gingivectomy 83% Scaling and root planing 8–80% Periodontal prophylaxis 0–40% Endodontics •intracanal instrumentation 0–31% •extracanal instrumentation 0–54% Endodontic Surgery •flap reflection 83% •periapical curettage 33% Toothbrushing 0–26% Dental flossing 20–58% Interproximal cleaning with toothpicks 20–40% Irrigation devices 7–50% Chewing 17–51%
|
TABLE 3. Prevalence of bacteremia arising after various types of dental procedures and oral activity. Reprinted from Seymour et al (15).
Source |
Imperiale et al,17 1990. United States |
Van Der Meer et al,18 1992. Netherlands |
Lacassin et al,19 1995. France |
Strom et al,20 1998. United States |
Duval et al, 21 2006. France |
Study Objective |
To determine whether pre-dental procedural antibiotic prophylaxis reduces the risk of infective endocarditis in persons with high-risk cardiac lesions. |
To asses the protective effect of antibiotic prophylaxis in subjects with native-valve and cardiovascular anomalies. |
To asses the relative risk of infective endocarditis associated with invasive procedures and the protective efficacy of antibiotic prophylaxis. |
To quantitate the risk for developing endocarditis in patients with underlying, at-risk cardiac conditions following invasive dental procedure. |
To estimate the risk of developing dental-procedural induced infective endocarditis in at- risk patients or received and did not receive antibiotic prophylaxis. |
Demographics and Pt characteristics. |
8 cases (native valve IE) and 24 controls. (without IE).
Pts were matched for age and high-risk cardiac lesion.
All experienced a dental procedure within 12 weeks of review. |
48 cases (native valve IE) and 200 controls (without IE).
Pts were matched for age, sex and cardiac condition.
All experienced a dental procedure 180 days prior to follow up interview.
|
37 cases (prosthetic or native valve IE) and 33 controls (without IE).
Pts were matched for age, sex and cardiac condition.
All experienced a dental procedure 3months prior to follow up interview.
|
29 cases (prosthetic or native valve IE) and 12 controls (without IE)
Pts were matched for age, sex, cardiac condition and geographic location.
All experienced a dental procedure 3months prior to follow up interview.
|
2805 patient population who experienced a dental procedure in previous 30days. Data reviewed to confirm the development of IE and administration of prophylactic antibiotic. Data was then extrapolated to estimate risk of developing IE and the protective efficacy of providing chemoprophylaxis to all high risk patients over 12 month period. |
Results |
1/8 case pts (13%) received antibiotics. 15/24 control pts (63%) received antibiotics. Matched Odds Ratio: .09 (CI upper limit of 0.93) (p=.025) Protective Efficacy 91%. |
8/48 case pts (16%) received antibiotics. 26/200 control pts (13%) received antibiotics. Stratified Odds Ratio: 0.51 (0.11-2.29). Protective Efficacy 49%. |
Any causative organism: 6/37 case pts (23%) received antibiotics. 6/33 control pts (27%) received antibiotics. Matched and Adjusted Odds Ratio: 0.2 (0-0.8) Protective Efficacy 20% Viridans Strep and Negative blood cultures 3/18 case pts (16%) received antibiotics 6/22 control pts (27%) received antibiotics Matched and Adjusted Odds ratio: 0.46 (0-0.9) Protective Efficacy 46%. |
24/29 case pts (83%) received antibiotics 3/12 control pts (25%) received antibiotics Unadjusted Odds Ratio: 0.5 (.01-9.6) Protective Efficacy 50%. Unadjusted Odds Ratio: 0.3 (.01-4.2) without abtc prophylaxis.
|
Risk of developing IE per 2.7million at risk patients not receiving prophylaxis: 1/46,000. Risk of developing IE with Antibiotic administration: 1/149,000 70% protective efficacy. Estimated number of IE cases prevented per 2.7million treated: 39-41
|
Study Limitations |
Excluded pts who died from IE. Administration of antibiotics was confirmed by patient recall when dental records were not available. Low IE incidence rate. |
Low IE incidence rate, large variation in predisposing cardiac condition severity. Results did not reach statistical significance. |
Low IE incidence rate, large variation in predisposing cardiac condition severity and inability to rule out confounding IE risk factors. Total number of dental procedures in the case group was greater then control group. |
Low IE incidence rate, and inability to rule out confounding IE risk factors.
|
Estimated risk extrapolated from geographically limited population of 2805 patients. |
Authors Conclusions |
Results suggest a IE protective benefit for pre-endodontic procedural chemoprophylaxis to patients of high-risk. The adverse risks and benefits of antibiotic prophylaxis need precisely defined. |
In a developed affluent and medically well organized country, complete compliance with endocarditis prophylaxis might prevent about 5 cases of IE per year. |
The efficacy benefit from antibiotic prophylaxis may reduce IE incidence by 5-10% or prevent 60-120 new cases of IE per year. |
Even at 100% effectiveness, prophylaxis would reduce IE incidence by 2.0 cases per 1,000,000 person-years.
Dental procedures are not a risk factor for endocarditis even in high-risk patients.
|
A very low number of IE cases would be prevented with a large population antibiotic prophylactic practice. The risk o fatal reactions to antibiotics, limits the use of prophylaxis to populations with the highest risk. |
TABLE 4. Characteristics of 5 Studies Addressing Antibiotic Prophylaxis for At-risk Patients.
Prosthetic and mechanical cardiac valves. Previous bacterial endocarditis. Complex cyanotic congenital heart disease (single ventricle states, transposition of the great arteries, tetralogy of Fallot).
Surgically constructed systemic pulmonary shunts or conduits. Acquired valvular dysfunction (rheumatic heart disease). Hypertrophic cardiomyopathy. Mitral valve prolapse with valvular regurgitation and/or thickened leaflets.
|
TABLE 5. Cardiac Conditions associated with increased IE acquisition risk. Adapted from Adnan et al (52).
FIGURE 1. Forest Plot of Antibiotic Efficacy as Reported by Case-Control studies
Conflicts of Interest:
None Declared
Adnan S. Dajani, MD; Alan L. Bisno, MD; Kyung J. Chung, MD; David T. Durack, MB, DPhil; Michael Freed, MD; Michael A. Gerber, MD; Adolf W. Karchmer, MD; H. Dean Millard, DDS, MS; Shahbudin Rahimtoola, MB, FRCP; Stanford T. Shulman, MD; Chatrchai Watanakunakorn, MD; Kathryn A. Taubert, PhD. Prevention of bacterial endocarditis. JAMA. 1990;264:2919-2922.
Agha Z, Lofgren RP, VanRuiswyk JV. Is antibiotic prophylaxis for bacterial endocarditis cost-effective? Med Decis Making. 2005;25:308-320.
Bahn SL, Goveia G, Bitterman P, Bahn AN. Experimental endocarditis induced by dental manipulation and oral streptococci. Oral Surg. 1978;45:549–550.
Bashore TM, Cabell C, Fowler V Jr. Update on infective endocarditis. Curr Prob Cardiol. 2006;31:274-352
Bender I B, Seltzer S, Yermish M. The prevalence of bacteremia in endodontic manipulation. Oral Surg Oral Med Oral Path. 1960;13:353-60.
Bor DH, Himmelstein DU. Endocarditis prophylaxis for patients with mitral valve prolapse. A quantitative analysis. Am J Med. 1984;76:711.
Carrion PK, Freedman LR. Experimental endocarditis. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970;42:394-410.
Conner HD, Haberman S, Collings CK, Winford TE. Bacteremias following
periodontal scaling in patients with healthy appearing gingiva. J Periodontol. 1967;38:466–472.
Crémieux AC, Saleh-Mghir A, Vallois JM, Muffat-Joly M, Devine C, Pocidalo JJ, Carbon C. Influence of the pre-treatment duration of infection on the efficacies of various antibiotic regimens in experimental streptococcal endocarditis. J Antimicrob Chemother. 1993;32:843–852.
Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, Gewitz MH, Shulman ST, Nouri S, Newburger JW, Hutto C, Pallasch TJ, Gage TW, Levison ME, Peter G, Zuccaro G Jr. Prevention of Bacterial Endocarditis : Recommendations by the American Heart Association. Circulation.1997;96:358 - 366.
Di Filippo S, F Delahaye, B Semiond, M Celard, R Henaine, J Ninet, F Sassolas, A Bozio. Current patterns of infective endocarditis in congenital heart disease. Heart. 2006;92:1490-1495.
Duval X, Alla F, Hoen B, Danielou F, Larrieu S, Delahaye F, Leport C, Briançon S. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis. 2006;42:102-7.
Frieling JT, Mulder JA, Hendriks T, Curfs JH, van der Linden CJ, Sauerwein RW. Differential induction of pro- and anti-inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrobial Agents and Chemotherapy.1997;41:1439-1443.
Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897-906.
Girard A, Cimochowski C, and Faiella J. The comparative activity of azithromycin, macrolides and amoxicillin against streptococci in experimental infections. J. Antimicrob. Chemother. 1993;31:29–37.
Heimdahl A, G Hall, M Hedberg, H Sandberg, P O Söder, K Tunér and C E Nord Detection and quantification by lysis-filtration of bacteremia after different oral surgical procedures. J of Clin Microbiol. 1990;28:2205-2209.
Helbling, A., Hurni, T., Mueller, U. R., Pichler, W. J. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940 000 inhabitants of the Swiss Canton Bern Clinical & Experimental Allergy. 2004;34:285-290.
Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
Imperiale TF, Horwitz RI. Does prophylaxis prevent post-dental infective endocarditis? A controlled evaluation of protective efficacy. Am J Med. 1990;88:131-136.
Kelkar P, James T. Current concepts: cephalosporin allergy. N Engl J Med. 2001;345:804-9.
Lacassin F, Hoen B, Leport C, Selton-Suty C, Delahaye F, Goulet V, Etienne J, Briançon S. Procedures associated with infective endocarditis in adults - A case control study. Eur Heart J. 1995;16:1968-1974.
Lee CE, Zembower TR, Fotis MA, Postelnick MJ, Greenberger PA, Peterson LR, Noskin GA.The incidence of antimicrobial allergies in hospitalized patients : implications regarding prescribing patterns and emerging bacterail resistance. Arch Int Med. 2000;160:2819-22.
Lin RY. A Perspective on penicillin allergy. Arch Intern Med. 1992;152:930-7.
Lockhart PB. An analysis of bacteremias during dental extractions: a
double-blind, placebo-controlled study of chlorhexidine. Arch Intern
Med. 1996;156:513–520.
Lockhart PB, Brennan MT, Kent ML, Norton HJ, Weinrib DA. Impact
of amoxicillin prophylaxis on the incidence, nature, and duration of
bacteremia in children after intubation and dental procedures. Circulation.
2004;109:2878 –2884.
Mazur N, Greenberger P, Regalado J. Clindamycin hypersensitivity
appears to be rare. Ann Allergy Asthma Immunol. 1999;82:443–5.
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-30.
Northrop PM, Crowley MC. The prophylactic use of sulfathiazole in transient bacteremia following the extraction of teeth. J Oral Surg. 1943;1:19-29.
Oliver R, Roberts GJ, Hooper L. Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2004, Issue 2.
Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin
North Am. 2003;47:665-679.
Prendergast, BD. The Changing face of Infective Endocarditis. Heart. 2006;92:879-885.
Rabinovich S, Evans J, Smith I, January L. A long-term view of bacterial endocarditis. Ann Intern Med. 1965;63:185-198.
Roberts G J, Gardner P, Simmons N A. Optimum time for detection of dental bacteremia in children. Int J Cardiol. 1992;35:311-315.
Roberts GJ. Dentists are innocent! Everyday" bacteremia is the real culprit: A review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatric Cardiology. 1999;20:317.
Rouse M S , J M Steckelberg, C M Brandt, R Patel, J M Miro, and W R Wilson. Efficacy of azithromycin or clarithromycin for prophylaxis of viridans group streptococcus experimental endocarditis. Antimicrob Agents Chemother. 1997;41:1673–1676.
Seymour, R A. Lowry R. Witworth J M. Martin M V. Infective endocarditis, dentistry and antibiotic prophylaxis: time for a rethink? British Dental Journal. 2000;189;610-616.
Strom B, Elias Abrutyn, Jesse A. Berlin, Judith L. Kinman, Roy S. Feldman, Paul D. Stolley, Matthew E. Levison, Oksana M. Korzeniowski, and Donald Kaye. Dental and cardiac risk factors for infective endocarditis: A population-based, case-control study. Ann Int Med. 1998;129:761-769.
Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, Levison ME, Korzeniowski OM, Kaye D. Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation. 2000;102:2842.
The International Collaborative Study of Severe Anaphylaxis. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiology and drug safety. 2003;12:195-202.
Van der Meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial endocarditis in The Netherlands II. Antecedent procedures and use of prophylaxis. Arch Intern Med. 1992;152:1869-1873.
Weinstein L, Brusch JL. Infective Endocarditis. New York, NY: Oxford University Press, 1996.
Wilson, W , Kathryn A. Taubert, Michael Gewitz, Peter B. Lockhart, Larry M. Baddour, Matthew Levison, Ann Bolger, Christopher H. Cabell, Masato Takahashi, Robert S. Baltimore, Jane W. Newburger, Brian L. Strom, Lloyd Y. Tani, Michael Gerber, Robert O. Bonow, Thomas Pallasch, Stanford T. Shulman, Anne H. Rowley, Jane C. Burns, Patricia Ferrieri, Timothy Gardner, David Goff, David T. Durack, and The Council on Scientific Affairs of the American Dental Association has approved the guideline as it relates to dentistry.Prevention of Infective Endocarditis. Guidelines From the American Heart Association. A Guideline From the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group Circulation published April 19, 2007.
First Published April 2012 - Copyright Priory Lodge Edcuation Limited
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-



