LARGE CELL NEUROENDOCRINE CARCINOMA OF THE LUNG IMMUNOHISTOCHEMICAL AND ELECTRON MICROSCOPIC CONFIRMATION
1. Dr. Salim Surani,
M.D.
2. Dr. Joseph Varon, M.D.
3. Richard Schacht RN, FNP
4. Salim Virjee
Key Words: Neuroendocrine, Pulmonary, Carcinoma, Large cell
Abbreviations:
FNA, fine needle aspiration. LCNC large cell neuroendocrine carcinoma. NSE, neuron specific enolase.
Summary
Primary pulmonary tumors exhibiting neuroendocrine differentiation are frequently encountered by clinicians. During the past decade primary malignant pulmonary neuroendocrine carcinoma has been diagnosed by cytology and immunohistochemistry. We report herein a case of large cell pulmonary neuroendocrine carcinoma in a 33 year old, non-smoker, female who was diagnosed by transthoracic fine needle aspiration and immunohistochemistry and electron microspy staining. The pulmonary mass invaded the mediastinum as well as the soft tissue outside the thoracic cage causing destruction of the ribs. The patient also developed a solitary extrathoracic metastasis to the right lacrimal gland.
Introduction
During the past decade primary malignant pulmonary neuroendocrine carcinomas have been diagnosed by fine needle aspiration ( FNA ) biopsy and immunohistochemistry. These pulmonary neuroendocrine cells share common features with cells of the classic peptide-secreting endocrine glands, scattered endocrine cells of the gastrointestinal and urogenital tracts, and cells of the nervous system1. We are reporting an unusual case of large cell neuroendocrine carcinoma of the lung that invaded the mediastinum and adjacent ribs and metastasized to the right lacrimal gland. A brief review of the literature on this entity is also included.
Case report
A 33 year-old female presented with complaints of anterior discomfort of the left chest for six weeks associated with dyspnea, night sweats, and a ten pound weight loss. She denied having fever. She had no history of tobacco, alcohol, or intravenous drug use. PPD and sputum for acid fast bacilli were negative. On physical exam the patient was afebrile, alert, oriented and in no distress. There was a fixed, hard, non-mobile eight by eight centimeter mass, under the left breast covered with normal appearing overlying skin. Remainder of the physical examination was normal.
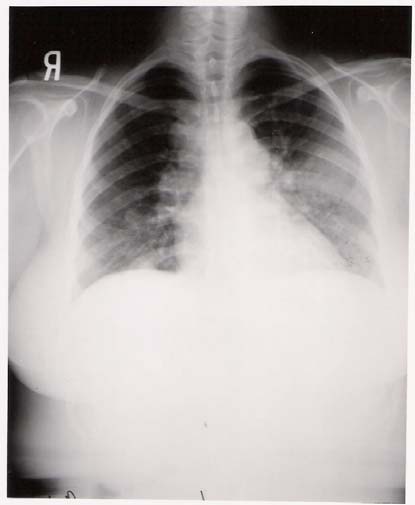
Laboratory tests including electrolytes, complete blood count and coagulation studies were within normal limits. Chest x-ray revealed bilateral hilar lymphadenopathy, mediastinal fullness, and a large solitary mass in the left lung field.
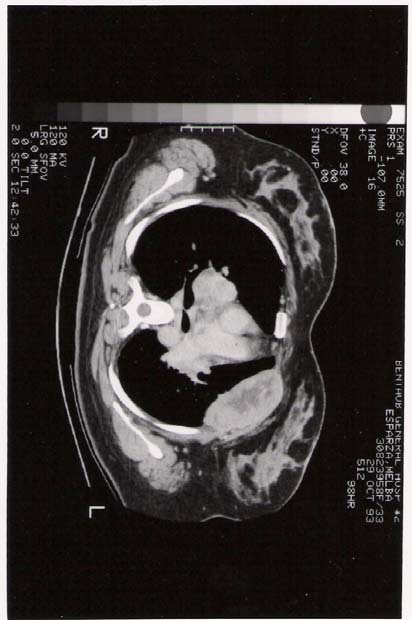
CT scan showed a large eight by eight centimeter, soft tissue mass with a hypodense center, involving the upper lobe of the left lung anteriorly. The mass extended to the adjacent thoracic wall, with complete destruction of the anterior portion of the left third an fourth ribs and involvement of the soft tissue outside the thoracic cage. Extensive perivascular, right paratracheal, precarinal, and bilateral hilar lymphadenopathy was also noted. Multiple pulmonary nodes of variable size, involved mainly the central portions of the lower lobes, bilaterally.
The CT scan of the abdomen revealed the liver and the adrenal glands to be normal. The CT scan of the orbit ( with contrast ) revealed an enhancing mass in the right lacrimal gland area with destruction of the overly bone laterally and the orbit roof. The left orbit was unremarkable. No intracranial abnormalities were seen.
The patient underwent fiber optic bronchoscopy which revealed no endobrachial lesion. The bronchial washing cytology was negative for malignant cells. Tumor cells from the fine needle aspiration ( FNA ) cytology of the mass was focally positive for neuron specific enolase ( NSE ), keratin ( AE1/AE3 ), S-100 protein and Leu-7. A biopsy of the mass in the right lacrimal gland were positive for Leu-7, NSE, and cytokeratin ( AE1/AE3 ). These results are consistent with maetastatic large cell neuroendocrine tumor of the lung. There was no immunoreactivity to antibodies against chromogranin, synaptophysin, vimentin, and BRST-2 ( breast marker ).
Electron microscopic studies revealed tumor cells with round to oval nuclei possessing minimal indentations of their nuclear membranes. In many cells, the nuclei showed a single peripherally marginated nucleolus as well as finely dispersed euchromatin. Intracytroplasmic organelles included lipid bodies, abundant glycogen, numerous mitochondria , and pleomorphic membrane-bound neuorsecretory granules, ranging in size from 300 to 700 nm. Interdigitating cell processes with terminal bar attachments also were observed.
Discussion
Primary tumors exhibiting neuroendocrine differentiation are frequently encountered by clinicians. A wide range of clinical manifestation occur with these tumors, from the extremely virulent small cell anaplastic carcinomas to the indolent clinical course of bronchial carcinoid tumors. These tumors exhibit certain morphologic and im munocytochemical characteristics, which allow them to be included as members of the amine precursors uptake and decarboxylation system, or dispersed neuroendocrine system. Such tumors are distinguished ultra structurally by the presence of variable numbers of dense core neuorsecretory granules and immonocytochemically by the production of small poly peptides2,3. The classification of pulmonary neuroendocrine tumors of the lung has evolved significantly over the past several years. Initially, only two major categories of pulmonary neuroendocrine tumors were recognized: the carcinoid and small cell lung carcinoma. Arrigoni et. al.4, was the first to describe the criteria for the atypical carcinoid tumor. Neuroendocrine tumors of the lungs have frequently been classified into threecategories: typical carcinoid, atypical carcinoid, and small cell carcinoma5. In 1991, Travis et. al. 6, utilizing electron microscopy reported a category of neuroendocrine tumor known as a large cell neuroendocrine carcinoma for the higher grade non-small cell carcinoma tumors, to be distinguished from large cell carcinoma with neuroendocrine features. Overall, statistically significantly survival data for these patients with large cell neuroendocrine carcinomas must await accumulation of a larger series of patients7. However, these tumors appear to be uncommon as only fifteen cases were collected by Volmer8,in twenty years. The data from Travis et. al. 6, suggest that the survival of the patients is intermediate between that observed in patients with atypical carcinoid and the small cell lung carcinoma. Travis et. al. 6, used ancillary techniques of immunohistochemistry, electron microscopy, and DNA analysis, and found no advantage in comparison with the conventional histological features in terms of prediction of prognosis of this large cell pulmonary endocrine tumor. The vast majority of large cell endocrine carcinomas of the lung are central, but they also may be peripheral. The mass is circumscribed and nodular and composed of large polygonal cells, having low nuclear/cytoplasmic ratio, coarse or vesicular nuclear chromatin, frequent nucleoli, high mitotic rate and frequent foci of necrosis. If Immunohistochemical studies or electron microscopy are not available, by light microscope, alone the tumor may be confused with a large cell carcinoma. The result of Immunohistochemical findings in a series of thirty five cases were positive as follows: neuron specific enolase ( 100% ), chromogranin ( 80% ), Leu-7 ( 40% ), and synaptophysin ( 40% )6.This is an aggressive neoplasm with prognosis approaching that observed in small cell carcinoma6. Our case represents a large cell neuroendocrine carcinoma which was diagnosed by trans thoracic fine needle aspiration, using Immunohistochemical techniques. In the series of thirty-five large cell carcinomas described by Travis et. al.6, all of the patients were smoker's as compared to our patient who was a nonsmoker. None of the previously reported patients had a maetastatic disease at the time of presentation, in contrast to our patient who also had metastases to the mediastinum, opposite lung parenchyma and right lacrimal gland. The only metastatic site (outside of the thoracic region) in our patient was to the right lacrimal gland which is quite unusual. The most common primary carcinomas that metastasize to the orbit and globe are breast cancer (40 %) an lung cancer (29%) in adults. A small number of carcinoids tumors maetastatic to the orbit have been reported10, as well as occasional cases of small cell carcinoma of the lung.Conclusions
In conclusion, our patient had an unusual presentation of a large cell neuroendocrine carcinoma, arising within the peripheral lung parenchyma, destroying adjacent ribs, as well as metastasizing to the right lacrimal gland causing destruction of the bony orbital roof. The patients youth, sex, and nonsmoking history are also interesting clinical features. As most patients with large cell endocrine tumors are in there seventh decade of life, four times more likely to be male, and are smokers with seventy-five percent having a forty pack a year plus history11.
References
1.
Arrigoni MG, Woolner LB, Bernatz PE. Atypical carcinoid tumors of the
lung. The Journal of Thoracic and Cardiovascular Surgery 1972; 64: 413-
421.
2.
Becker KL. The coming age of a bronchial epithelial cells. American
Review of Respiratory Disease; 148: 1166-1168.
3.
Carter D, Yesner R. Carcinomas of the lung with neuroendocrine
differentiation. Seminars in Diagnostic Pathology 1985; 3: 235-254.
4.
Fielder DB. Neuroendocrine tumors of the lung: recent developments in
histopathology .Current Opinion in Pulmonary Medicine 2002; 8: 275-
280
5.
Gould VE. Linnolia RI, Memoli VA, Warren WH. Neuroendocrine cells
and neuroendocrine neoplasms of the lung. Pathology Annual 1983; 18:
287-330.
6.
Gould VE, Memoli V, Chejfic G, Johannesses JV. The APUD cell system
and its neoplasms: Observations on the significance and limitations of the
concept. Surgical Clinics North America 1979; 59: 93-108.
7.
Shelter DJ. Font RL, Ordonez N, El-Naggar A, Boniuk M. A
clinicopathologic study of three carcinoid tumors metastatic to the orbit.
Ophthalmology 1990; 97: 257-264.
8.
Travis WD, Linnolia RI, Tsokos MG, Hitchcock CL, Culter GB, Nieman
L, Chrousos G, Pass H, Doppman J. Neuroendocrine Tumors of the lung
with proposed criteria for larger cell neuroendocrine carcinoma: An
ultrastructral, Immunohistochemical, and flow cytometric study of 35
cases. American Journal of Surgical Pathology 1991; 15: 529-553.
9.
Volmer RT. The effect of cell size on the pathologic diagnosis of small
and large cell carcinomas of the lung. Cancer 1982; 50: 1380-1383.
10.
Warren WH, Faber LP, Gould VE. Neuroendocrine neoplasms of the lung:
A clinicopathologic update. The Journal of thoracic and cardiovascular
surgery 1989; 98: 321-332.
First Published October 2005
AUTHORS
1. Dr. Salim Surani, M.D.2. Dr. Joseph Varon, M.D.
3. Richard Schacht RN, FNP
4. Salim Virjee
Key Words: Neuroendocrine, Pulmonary, Carcinoma, Large cell
Abbreviations:
FNA, fine needle aspiration. LCNC large cell neuroendocrine carcinoma. NSE, neuron specific enolase.-
Assistant Professor Texas A&M, Corpus Christi, Texas
-
Professor of Medicine, University of Texas, Houston, Texas
-
Corpus Christi, Texas,
-
Corpus Christi, Texas
Correspondence to:
Dr. Salim Surani MD
613 Elizabeth street
Physicians Tower suite 813
Corpus Christi, Texas, 78404
Phone: (361) 885-7722
Fax: (361) 885-7792
Email: srsurani@hotmail.com
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ŠPriory Lodge Education Ltd 1994-

