Adrenaline use in anaphylaxis: friend or foe?
Dr Andrew Bentley
Consultant in Respiratory and Critical Care MedicineSouth Manchester University Hospital Trust Wythenshawe Hospital Manchester, M23 9LT, UK
Dr Dermot RyanGPIAG Clinical Research Fellow
Department of Primary Care, University of Aberdeen
General Practitioner, Woodbrook Medical Centre, Loughborough,
LE11 1NH,
UK
Dr David Luyt
Consultant in Paediatric Medicine,
Leicester Royal Infirmary,
Leicester,
LE1 5WW,
UK
Abstract
Anaphylaxis creates fear and uncertainty throughout the medical profession and general public alike. It occurs unexpectedly and may progress rapidly in patients of all ages, often in the young and otherwise healthy. If treated inappropriately, or not treated at all, it may, in rare cases, prove fatal. Rapid diagnosis is essential and dependent on clinical recognition alone. On recognition, immediate injection of intramuscular adrenaline is the treatment of choice, the response to which is often dramatic and potentially life-saving. The use of adrenaline in the treatment of acute anaphylaxis is well established. However, evidence suggests that adrenaline auto-injectors are under-used in severe reactions, related in part to a reluctance to prescribe them. This has been highlighted in a recent report conducted by The Anaphylaxis Campaign (Uguz et al, 2005). One of the major challenges, with respect to diagnosis and management of anaphylaxis, is the lack of a standard recognised working definition. The purpose of this article is to review anaphylaxis and intramuscular adrenaline administration, particularly for first medical responders.
What is anaphylaxis?
In 1902, Charles Richet and Paul Portier first described the phenomenon of anaphylaxis, which literally means 'against protection', aboard Prince Albert of Monaco's yacht in the Mediterranean. They reported on their attempts to immunise dogs against jellyfish stings (Portuguese Man-of-War), which resulted in the sudden death of a number of the animals. Richet subsequently won the Nobel Prize for physiology or medicine in 1913 for his work on anaphylaxis (Nobel Lectures, 1901–1921).
The European Academy of Allergy and Clinical Immunology defines anaphylaxis as 'a severe, life-threatening, multiple-organ hypersensitivity, often dominated by severe asthma and hypotension'. The clinical syndrome of anaphylaxis may involve cutaneous, respiratory, cardiovascular or gastrointestinal symptoms and signs
(Figure 1).
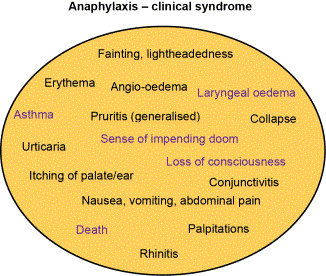
Figure 1. The constellation and complexity of symptoms of anaphylaxis requiring rapid interpretation. These may also be complicated by panic or fainting.
How does anaphylaxis present?
A clinical presentation of anaphylaxis in its extreme or classical form is easily recognised. In reality, it is often far more difficult to identify, with variable target organ involvement and expression of symptoms. They can be categorised as mild, moderate or severe (Box 1) (Brown, 2004).
Clinical diagnosis of anaphylaxis involves the recognition of one or both of the two severe features of airway compromise (laryngeal oedema and/or asthma) and hypotension (collapse, loss of consciousness, fainting). This is clearly highlighted in the clinical algorithm of the Resuscitation Council's (UK) guideline (Project Team of the Resuscitation Council, UK, 2005). More complete lists of clinical symptoms are also available (Sampson et al, 2005). Identifying these severe features aids clinical diagnosis. Difficulty arises in the interpretation of the symptoms and signs. For example, urticaria and angio-oedema, in the absence of laryngeal oedema or airway involvement, is not anaphylaxis.
The aetiology of anaphylaxis
From an immunological perspective, anaphylaxis can be defined as an immediate systemic reaction caused by the rapid release of potent mediators from tissue mast cells and peripheral basophils (AAAI, 1998). Allergen-driven cross-linking of receptor-bound IgE activates mast cells and basophils, to release their mediators (Figure 2) (Galli et al, 2005).
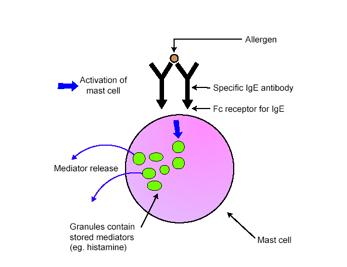
Figure 2. Activation of mast cells in response to allergens (Galli et al, 2005).
Anaphylaxis can be provoked by numerous agents, or allergens, most usefully categorised into drug or non-drug causes (Table 1).
Table 1. The causes of anaphylaxis
| Non-drug causes | Drug causes |
| Foods –any, but notably nuts, milk, egg, seafood, fish | Antibiotics – especially penicillin/ cephalosporins |
Insect stings – wasps, bees |
Anaesthetic agents – neuromuscular blocking drugs reported most commonly |
Latex rubber – health care workers, spina bifida patients (multiple medical procedures) |
Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) |
Exercise induced – often with concomitant food (e.g., wheat) reaction (in up to 50% of cases) |
Intravenous contrast media, blood products and intravenous fluids (colloidal starch) |
| Idiopathic – up to 20% of cases (in adults; rare in children), but this is a diagnosis of exclusion | Opioid analgesics |
| ACE inhibitors |
For anaphylaxis to occur, a patient has usually been previously sensitised to the allergen. The initial sensitisation is a complex process, involving multiple cell types and mediators, and is also affected by environmental and genetic factors.
The immunobiology and pathophysiology of anaphylaxis are basically the same, irrespective of the initial trigger, although there may be subtle differences in the responses.
The term 'anaphylactoid' has been used to represent the identical clinical pictures seen as a result of the degranulation of mast cells and basophils, but which are not mediated through IgE. The treatment for both of the mechanisms is identical, and the misinterpretation of anaphylactoid reactions has resulted in fatal re-exposure of the patient to the allergen concerned (Fischer, 1995).
The release of cell mediators during anaphylaxis leads to end-organ responses in the skin, respiratory, cardiovascular, gastrointestinal, and central nervous systems. The combined physiological effects of these mediators contribute to a multi-organ 'hypovolaemic-distributiveÓshock,whichinvolves:
¤ Smooth muscle contraction, leading to bronchoconstriction and abdominal cramps
¤ Vasodilatation, leading to flushing, urticaria, hypotension and a reduced level of consciousness
¤ Increased capillary permeability, leading to angio-oedema and laryngeal oedema
¤ Activation of vagal pathways, leading to bradycardia and neurocardiogenic syncope.
The rapidity of onset of the severe symptoms and their presentation differ, depending on the causal agent. In a study of fatal anaphylaxis, the median time to cardiac or respiratory arrest was 30 minutes for food allergens, 15 minutes for insect venom and 5 minutes for medications or radiological contrast reagents (Pumphrey, 2000). Allergens such as intravenous drugs or insect stings more commonly result in circulatory collapse and shock. Ingested allergens in food-related anaphylaxis are more likely to produce symptoms of upper airway obstruction, or respiratory compromise and shock is less common, unless the patient stands up. In keeping with this view, an adjunct to the treatment of acute anaphylaxis, presenting with features of circulatory collapse, is to ensure the patient is lying down with their feet elevated, thereby aiding venous return and maintaining cardiac filling pressures (Brown, 2005).
An anaphylactic reaction is not necessarily a discrete event. Biphasic reactions are well described, as are delayed onset, protracted or persistent reactions (Starks and Sullivan, 1986; Lieberman, 2005). These factors should be taken into consideration in the observation period, following resolution of the initial event. They can occur with sufficient frequency as to recommend an overnight or 24-hour waiting period before discharge (Project Team of the Resuscitation Council, UK revised anaphylaxis guideline May 2005). Furthermore, fatal reactions are more likely to occur in individuals with concomitant asthma, and this should be recognised in both food-related anaphylaxis and in reactions to insect stings (Sampson et al, 1992; Settipane et al, 1980; Bock et al, 2001; Lee and Greenes, 2000).
Anaphylaxis: epidemiology and mortality
The true incidence of anaphylaxis is unknown. Accurate epidemiological studies have been hampered by the lack of an agreed definition of anaphylaxis, the different population groups studied and under-recognition and under-reporting. Estimates range from 10–30 cases per 100,000 population per year, with approximately 1 in 400–2000 emergency attendees (Peng and Jick, 2004; Stewart and Ewan, 1996; Yocum et al, 1999; Klein and Yocum, 1995; Brown et al, 2001). There has been a dramatic ten-fold increase in hospital admissions for anaphylaxis and other severe allergic reactions in the last decade (Sheikh and Alves, 2000).
Anaphylaxis remains an important and avoidable cause of death. Data from a UK survey of fatal anaphylaxis in individuals over 16 years of age reported 20 deaths per year: 10 related to drugs, 5 to insect stings and 5 to foods (Pumphrey, 2000). In two thirds of cases, the fatal reaction was the first reaction, but not necessarily at the first exposure. A number of individuals reportedly died, despite optimal clinical management. Pumphrey studied post-mortem findings on 56 cases of fatal anaphylaxis (19 caused by stings, 16 by food and 21 by drugs or contrast media) and found macroscopic features of asthma in 15 of the cases, petechial haemorrhages in 10, laryngeal oedema in 23 and no specific features in 23 (Pumphrey and Roberts, 2000).These findings may reflect the differences in clinical presentation between asthma/upper airway obstruction and catastrophic circulatory collapse.
Treatment of anaphylaxis: adrenaline
Adrenaline treatment: route of administration
Adrenaline (a hormone and neurotransmitter secreted by the adrenal gland) is the mainstay of treatment for anaphylaxis and is central to the Resuscitation Council's (UK) guideline (Project Team of the Resuscitation Council, UK, 2005). Adrenaline injected by an auto-injector into the anterolateral aspect of the thigh is the gold standard of care in the management of acute anaphylaxis. The term 'first responder' refers to the individual who initially recognises and initiates the treatment for anaphylaxis, for example, the individuals themselves, family members and community nurses.
The routes of administration of adrenaline for anaphylaxis that have been studied are by intramuscular, subcutaneous or intravenous injection, or by inhalation. These studies conclude that the administration route of choice for first time responders is by intramuscular injection into the antero-lateral thigh muscle. The subcutaneous route can no longer be recommended, as a study in children showed absorption to be delayed and variable (Simons et al, 1998). The average time to maximum plasma adrenaline concentration using the intramuscular route was 8 minutes, compared with 34 minutes using the subcutaneous route. Furthermore, the average maximum plasma concentration of adrenaline was significantly higher using the intramuscular route (Simons et al, 2001a).
Inhaled adrenaline also cannot be recommended for the treatment of anaphylaxis. In a study in children, those treated with adrenaline inhalers had blood adrenaline levels no higher than a control group treated with placebos (Simons FER, 2000b).Furthermore, the bad taste of adrenaline actually prevented the children from taking the correct number of inhalations. It has also been suggested that inadequate dosing may occur in some women, particularly those with a BMI >30, because of a skin-to-muscle distance greater than the depth of the needles (1.43 cm) used (Song et al, 2005).
Intravenous adrenaline is not indicated in first-responder management of acute anaphylaxis, although it has a clear role in other forms of anaphylaxis. These include anaesthetic-related anaphylaxis and refractory anaphylaxis, especially in cases not responding to intramuscular adrenaline, in a clinical area where cardiac monitoring and full resuscitation facilities are available. Although intravenous adrenaline has been associated with fatal cardiac arrythmias and myocardial infarction, these cases have been associated with too rapid injection, undiluted doses, or excessive doses (Fischer, 1995; Pumphrey, 2000; Brown, 2001; Montanaro and Bardana, 2002). To minimise these adverse effects, the use of intravenous adrenaline is now recommended at a dilution of 1:10,000 and is titrated in 1 ml aliquots in adults (Project Team of the Resuscitation council, UK, 2005).
Dose considerations
The recommended dose of adrenaline in the treatment of acute anaphylaxis in adults is 0.5mg administered intramuscularly (Project Team of the Resuscitation council, UK, 2005). Repeat doses may be considered, should the individual not respond, or deteriorate clinically, after the first dose. It would be preferable to give this dose where both medical help and monitoring is available. Adrenaline auto-injectors deliver either 0.3 mg or 0.15 mg adrenaline to adults and children respectively. They can be regarded as equivalent to the 0.25 mg and 0.12 mg doses that are more generally recommended (table 2).For first responders these doses are entirely appropriate as most patients only require one dose from an auto-injector in response to a reaction. Because of the lower dose however, it is advisable to always prescribe two adrenaline auto-injectors, in case of an inadequate or partial response, or early relapse (biphasic reaction).
In the case of children, although a precise dose administered, based on weight, is most accurate, it lacks day-to-day applicability for first-responders. For this reason, a fixed dose, based on age, was devised and modified (Project Team of the Resuscitation Council (UK) revised May 2005) (Table 2).
Table 2. Recommended doses of adrenaline, based on age
Age |
Dose |
Auto-injector equivalents |
12 years & above |
Up to 0.5 mg IM |
2 doses of adult auto-injector; 0.25 mg if child is small or pre-pubertal |
6–12 years |
Up to 0.25 mg IM |
2 doses of junior auto-injector |
>6months–6 years |
0.12 mg IM |
One dose of junior auto-injector |
<6 months |
0.05 mg IM (0.05 ml 1:1000 solution) |
N/A |
There has been a call for additional fixed dose formulations for use in the community, to allow more precise dosing in young children (Simons et al, 2001b; Simons et al, 2002). If auto-injectors are out of date, the bioavailability of adrenaline is significantly reduced. However, the potential benefit from suboptimal adrenaline dosing, compared with no adrenaline at all, is far greater, providing there is no discolouration or any precipitates apparent (Simons et al, 2000a).
Efficacy of adrenaline treatment
Double blind placebo controlled trials of adrenaline use in anaphylaxis are obviously not feasible, other than in animal models. However, there is universal acceptance of the value of intramuscular adrenaline, based on clinical observation and interpretation of the pathophysiology.
Adrenaline acts through adrenoreceptors, having both excitatory and inhibitory physiological effects (Table 3) (Brown AFT (1998). The physiological benefits of adrenaline in anaphylaxis are due to the increase in peripheral vascular resistance by vasoconstriction. As a result, there is an improvement in blood pressure, counteracting peripheral vasodilatation, as well as a reduction in angio-oedema. Coronary perfusion is increased as blood pressure improves, although inappropriate administration of adrenaline, such as by the intravenous route or with too high a dose, can result in coronary artery vasoconstriction and an increase in myocardial oxygen consumption. Adrenaline-induced bronchodilation counteracts the airway smooth muscle contraction and airway narrowing seen in anaphylaxis.
Table 3: Adrenoreceptors
α1 – vasoconstriction and relaxation of GI tract
α2 – platelet aggregation and reduction in noradrenaline release from nerve terminals
β1 – inotropic and chronotropic cardiac effects and relaxation of GI tract
β2 – bronchodilatation, increase in noradrenaline release from nerve terminals, increase in intracellular cyclic adenosine monophosphate (cAMP) production in mast cells and basophils, reduction in the release of cellular mediators (Brown, 1998)
Safety of adrenaline treatment
Intramuscular adrenaline, given into the lateral thigh, is safe in doses recommended by the Project Team of the Resuscitation Council (UK) (revised May 2005). Anaphylaxis or angio-oedema of the upper airway is life threatening, and avoiding or delaying appropriate treatment can lead to death (Sampson et al, 1992). Much emphasis has been placed on the dangers of adrenaline administration, especially when given outside the context of life threatening allergic reactions (Johnstone et al, 2003). This unfortunately fuels concerns about auto-injectors, heightens the anxiety of first-responders, including community nurses and parents and possibly contributes to their under-use.
Inappropriately diluted (1 in a 1000) intravenous adrenaline is cardiotoxic and dangerous (Jowitt, 2000; Fisher, 1992). Intramuscular adrenaline (1:1000) in doses of 0.01–0.4 mg/kg is not associated with clinically significant cardiotoxicity, even if given inadvertently in the absence of acute anaphylaxis. A study of the pharmacokinetics of intramuscular adrenaline delivered via auto-injector, showed a high peak level at 5 minutes, with continued elevation to at least 20 minutes. If a repeat IM injection is required, it has been recommended to be given with medical monitoring, where possible (Simons et al, 1998).
The benefits if IM administration of adrenaline greatly outweigh the risks, even in those with greater susceptibility to its adverse consequences e.g., the elderly and individuals with poorly controlled hypertension, known ischaemic heart disease and arteriopathies (Pumphrey, 2000; Project Team of the Resuscitation council, UK, 2005; Johnstone et al, 2003). Prompt, early monitoring, and avoidance of adrenaline overdosage, is perhaps one of the more important issues in this group of patients with significant comorbidities.
Drug interactions
Tricyclic antidepressants and monoamine oxidase inhibitors can potentiate the effect of adrenaline, and therefore contribute to an increased risk of cardiac arrhythmia. Cocaine sensitises the heart to catecholamines and thereby potentiates the effects of adrenaline. β blockers may decrease the effectiveness of adrenaline, and also risk increasing the effects of unopposed α adrenoreceptor and reflex vagal activity, resulting in bradycardia, hypertension, coronary vasoconstriction and bronchoconstriction. The dose of adrenaline may be halved in these cases. A first-responder can achieve this by using a junior 0.15 mg adrenaline auto-injector. If this is not possible, 0.3 mg via an adult auto-injector should be given and consideration given to doubling the length of time between a repeat dose from 5 to 10 minutes, depending on the clinical response. β blockers, which include eye drops, should, if possible, be discontinued in individuals at risk of anaphylaxis (Lang, 1995; Ellis and Day, 2003; Moneret-Vautrin et al, 1993).
Adrenaline auto-injectors
Adrenaline must be injected promptly at the first evidence of respiratory or cardiovascular compromise as signs of anaphylaxis. Even then, 10% of cases may not be reversible (Bock et al, 2001). Evidence supporting the early use of adrenaline emphasises that the more advanced the reaction, the less likely adrenaline is to reverse it (Bautista et al, 2002).
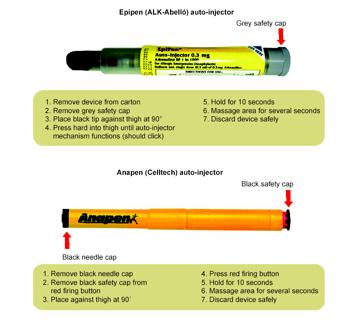
Figure 3: Autoinjector devices, reproduced with permission from McLean-Tooke, 2003, BMJ Publishing.
Adrenaline auto-injectors (Figure 3) have enabled adrenaline to be given by first-responders. Although there is no published evidence to show a reduction in mortality due to anaphylaxis, with adrenaline auto-injectors, there is compelling support for their early availability. Delayed administration of adrenaline can undoubtedly result in response failures and fatal outcomes (Sampson et al 1992, Soreide et al 1998, Pumphrey 2000).
If a diagnosis of anaphylaxis is made in primary care, it is prudent to prescribe an auto-injector as part of a complete management package. This should include explicit instructions on how and when to use it. Correct auto-injector technique should be demonstrated to the patient or carer and their technique observed using auto-injector training devices.
A complete management package should comprise explicit instructions as to what to do after using an auto-injection, for example, taking a non-sedating antihistamine, 30 mg of oral prednisolone and proceeding to the nearest emergency department for a period of observation and assessment. This is essential in order to monitor and treat early clinical deterioration in the event of initial inadequate treatment and to treat a subsequent biphasic reaction (Table 4).
McLean-Tooke and colleagues proposed a detailed algorithm for identifying patients who may benefit from an adrenaline auto-injetcor, irrespective of the aetiology of the anaphylaxis (McLean-Tooke et al, 2003). A more practical and pragmatic approach is taken in Table 4, by highlighting the 'at riskÓ features for anaphylaxis as key indicators for the prescription of an adrenaline auto-injector.
Table 4. 'At risk' patients: Indications for an adrenaline auto-injector?
![]()
á Previous severe allergic reaction involving respiratory (airway narrowing or laryngeal oedema) and/or haemodynamic compromise (hypotension, collapse, reduced level of consciousness)á Mild or moderate allergic reaction to low-dose exposure of allergen (e.g., peanut/nut allergy)á Previous allergic reaction with coexisting asthma, especially in relation to food allergyá Venom anaphylaxis with previous severe allergic reaction with or without co-existing asthmaá Drug-related anaphylaxis (e.g., history of severe reactions to penicillin/cephalosporin in patients with an essential requirement for regular courses of antibiotics)á Idiopathic anaphylaxis
Barriers to adrenaline use
Although the prescription of adrenaline auto-injectors has increased over the last few years, epidemiological data suggest that they are still under-prescribed and under-used despite fears over safety and inappropriate prescription (Unsworth, 2001). When patients are assessed in the clinic, adrenaline should remain the default treatment option, with emphasis being made on education and training covering administration and technique. If there is an active decision not to prescribe adrenaline, this should be fully explained and agreeable to all parties concerned.
The need for clear guidance on both the recognition of anaphylaxis and the indications for auto-injector adrenaline are crucial in the early management of the condition. A broad training package is essential and should focus not only on the administration of adrenaline, but also on wider aspects of education, recognition of an allergic reaction and prevention (Vickers et al, 1997).This is particularly relevant in a community setting, where the diagnosis often needs to be made by inexperienced non-medical first responders in a highly stressful situation. Barriers to the use of adrenaline, even when it has been prescribed, include the failure to recognise the symptoms of anaphylaxis; a belief that the reaction was mild; proximity of a hospital; spontaneous recovery after a previous episode; a reliance on oral antihistamines; fear of pain from the injection; and concerns about the side effects of adrenaline.
It is recognised that only 50–75% of patients prescribed auto-injectors carry them at all times (Drug Ther Bull, 2003; Unsworth, 2001; Goldberg, 2000; Sicherer, 2001). Furthermore, only 30–40% of individuals can correctly demonstrate the method of administration, and carrying an auto-injector does not mean it will be used. In one study, only 29% of children with recurrent anaphylaxis were actually treated with their adrenaline. However, the need for adrenaline on arrival to hospital was reduced in those who received an appropriate dose by auto-injector (Gold et al, 2000). In addition, many patients state they would seek medical advice or ask another person, as they would not want to self-administer adrenaline.
Unfortunately, the anxiety and uncertainty exhibited by patients and relatives is not helped by a lack of knowledge of anaphylaxis and allergic conditions within the medical profession. Most doctors in primary and secondary care are uncertain about the correct method for the use of adrenaline auto-injectors (Sicherer, 2001; Hayman et al, 2003). Instruction in their use provided by an 'allergy specialist' can improve the proper injection technique (Goldberg et al, 2000; Sicherer, 2001).
Discussion
Anaphylaxis remains a significant cause of morbidity and mortality. Despite the lack of a recognised definition, the provision of a practical guideline has been invaluable in its emergency management (Project Team of the Resuscitation Council, UK, 2005). Widespread dissemination of this knowledge through, for example, advanced life support courses, has put anaphylaxis into focus for providers of emergency care. It is highlighted as a 'peri-arrest' situation, in which prompt treatment may prevent cardiac arrest.
Beyond this point in the follow-up management, there is a huge gap in the provision of care. Unfortunately, this is precisely where aspects of education and training for patients and their families at risk of anaphylaxis need to be provided. This stems from a lack of basic knowledge of allergy within the medical profession and a lack of provision of primary and secondary care services for the appropriate investigation and co-ordinated management of these at-risk patients. There is little medical school and postgraduate training in anaphylaxis, as indicated by the demand for an article reviewing the key points of a primary care consultation of a patient presenting with a recent history of anaphylaxis (Sheikh and Walker, 2005). There are only six recognised allergy centres within the UK offering a complete range of services with full-time allergists. It is perhaps not surprising that, against this background, the provision of support for the patient at risk of anaphylaxis is limited. The outcomes from the Department of Health review of Allergy Services are eagerly awaited.
More widespread use of adrenaline auto-injectors must be combined with closer liaison between primary care, acute, community and education services. These are essential for the successful management of patients at risk of anaphylaxis, many of whom are children. In order to adequately provide for this group of patients, there needs to be a fundamental improvement and investment in clinical allergy services, with focus on improving both the knowledge and the clinical service (Ryan et al, 2005).
Conclusions and summary
- Anaphylaxis is a severe, life-threatening reaction that can affect all age groups
- The severity of previous reactions does not predict the severity of subsequent reactions
- Intramuscular adrenaline is the first-line treatment for anaphylaxis, with intravenous adrenaline reserved for unresponsive anaphylaxis or circulatory collapse
- Early use of adrenaline in anaphylaxis is associated with improved outcomes
- Any patient with a systemic allergic reaction should be considered for an adrenaline auto-injector with emphasis on identifying at risk features
- There is a clear need to improve the education of both patient and physician on the use of, and indications for, adrenaline auto-injectors
AcknowledgementsThe publication of this paper was supported by ALK-Abello Ltd.
References
- AAAI. (1998) American Academy of Allergy Asthma and Immunology (AAAAI) Joint task force.
- Bautista, E, Simons, F E, Simons, K J, Becker, A B, Duke, K, Tillett, M, Kepron, W, Mink, S N. (2002) Epinephrine fails to hasten haemodynamic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Appl Immunol. 128:151–164.
- Bock, S A, Munoz-Furlong, A, Sampson, H A. (2001) Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 107:191–193.
- Brown, A F T. (1998) Therapeutic controversies in the management of acute anaphylaxis. J Accident Emerg Med. 15:89–95.
- Brown, A F, McKinnon, D, Chu, K. (2001) Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol. 108:861–866.
- Brown, S. (2004) Clinical Features and severity grading of anaphylaxis. J Allergy Clin Immunol. 114: 371–376.
- Brown, S G. (2005) Cardiovascular aspects of anaphylaxis; implications for treatment and diagnosis. Curr Opin Allergy & Clin Immunol. 5(4):359–364.
- Ellis, M D, Day, J H. (2003) The role of epinephrine in the treatment of anaphylaxis. Curr Allergy Asthma Rev. 3:11–14.
- Ewan, P, Clark, A T. (2005) Efficacy of a management plan based on severity assessment in longitudinal and case-controlled studies of 747 children with nut allergy: proposal for good practice. Clin Exp Allergy. 35(6):751–756.
- Fisher, M. (1992) Treating anaphylaxis with sympathomimetic drugs. BMJ. 305:1107–1108.
- Fisher, M. (1995) Fortnightly Review: treatment of acute anaphylaxis. BMJ. 311:731–733.
- Galli, S, Kalesnikoff, J, Grimbaldeston, M A, Piliponsky, A M, Williams, C M, Tsai, M. (2005) Mast cells as 'tunableÓ effector and immunoregulatory cells: recent advances. Ann Rev Immunol. 23:749–786.
- Gold, M S, Sainsbury, R. (2000) First aid anaphylaxis management in children who were prescribed an epinephrine auto-injector device (EpiPen). J Allergy Clin Immunol. 106:171–176.
- Goldberg, A, Confino-Cohen, R. (2000) Insect sting-inflicted systemic reactions: attitudes of patients with insect venom allergy regarding after-sting behaviour and proper administration of adrenaline. J Allergy Clin Immunol. 106:1184–1189.
- Golden, D B, Marsh, D G, Kagey-Sobotka, A, Freidhoff, L, Szklo, M, Valentine, M D, Lichtenstein, L M. (1989) Epidemiology of insect venom sensitivity. JAMA. 262:240–244.
- Hayman, G, Bansal, J A, Bansal, A S. (2003) Knowledge about using auto-injectable adrenaline: a review of patients' case notes and interviews with general practitioners. BMJ. 327:1328.
- Johnstone, S L, Unsworth, J, Gompels, M M. (2003) Adrenaline given outside the context of life threatening allergic reactions. BMJ. 326:589–590.
- Jowitt, N I. (2000) Speed of treatment affects outcome in anaphylaxis. BMJ. 321:571.
- Klein, J S, Yocum, M W. (1995) Underreporting of anaphylaxis in a community emergency room. J Allergy Clin Immunol. 95:637–638.
- Lang, D M. (1995) Anaphylactoid and anaphylactic reactions. Hazards of beta-blockers. Drug Safety. 12(5):299–304.
- Lee, J M, Greenes, D S. (2000) Biphasic anaphylactic reactions in paediatrics. Paediatrics. 106(4):762–766.
- Lieberman, P. (2005) Biphasic anaphylactic reactions. Ann Allergy Asthma Immunol. 95(3):217–226.
- Macdougall, C F, Cant, A J, Colver, A F. (2002) How dangerous is allergy in childhood? The incidence of severe fatal allergic reactions in UK and Ireland. Arch Dis Child. 86:236–239.
- McLean-Tooke, A, Bethune, C A, Fay, A C, Spickett, G P. (2003) Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ. 327:1332–1335.
- Moneret-Vautrin, D A, Kanny, G, Faller, J P, Levan, D, Kohler, C. (1993) Severe anaphylactic shock with cardiac arrest caused by coffee and gum Arabic potentiated by beta-blocking eye drops. Rev Intern Med. 14(2):107–111.
- Montanaro, A, Bardana, E J Jr. (2002) The mechanisms, causes and treatment of anaphylaxis. J Invest Clin Immunol. 2:2–11.
- Nobel Lectures in Physiology or Medicine 1901–1921 (1967). Elsevier, Amsterdam.
- Peng, M M, Jick, H. (2004) A population-based study of the incidence, cause and severity of anaphylaxis in the UK. Arch Intern Med. 164(3):317–319.
- Project Team of the Resuscitation Council (UK) (Revised May 2005). The emergency medical treatment of anaphylactic reactions for first medical responders and for community nurses. www.resus.org.uk.
- Pumphrey, R S. (2000) Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 30:1144–1150.
- Pumphrey, R S, Roberts, I S. (2000) Postmortem findings after fatal anaphylactic reactions. J Clin Pathol. 53(4):273–276.
- Ryan, D, et al. (2005) Management of allergic problems in primary care: time for a rethink? PCRJ. 14:195–203.
- Sampson, H A, Mendelson, L, Rosen, J P. (1992) Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 327:380–384.
- Sampson, H A, Munoz-Furlong, A, Bock, S A, Schmitt, C, Bass, R, Chowdhury, B A, Decker, W W, Furlong, T J, Galli, S J, Golden, D B, Gruchalla, R S, Harlor, A D Jr, Hepner, D L, Howarth, M, Kaplan, A P, Levy, J H, Lewis, L M, Lieberman, P L, Metcalfe, D D, Murphy, R, Pollart, S M, Pumphrey, R S, Rosenwasser, L J, Simons, F E, Wood, J P, Camargo, C A Jr. (2005) Symposium on the definition and management of anaphylaxis : summary report. J Allergy Clin Immunol. 115:584–591.
- Settipane, G, Chafee, F H, Klein, D E, Boyd, G K, Sturam, J H, Freye, H B. (1980) Anaphylactic reactions to Hymenoptera stings in asthmatic patients. Clin Allergy. 10:659–665.
- Sheikh, A, Alves, B. (2000) Hospital admissions for acute anaphylaxis: time trend study. BMJ. 320(7247):1441.
- Sheikh, A, Walker, S. (2005) 10-minute consultation – anaphylaxis. BMJ. 331:330.
- Sicherer, S. (2001) Self injectable epinephrine: no size fits all! Ann Allergy Asthma Immunol. 86:597–598.
- Simons, F E, Roberts, J R, Gu, X, Simons, K J. (1998) Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 101:33–7.
- Simons, F E, Gu, X, Simons, K J. (2000a) Outdated Epipen and Epipen Jr auto-injectors: past their prime? J Allergy Clin Immunol. 105(5):1025–1030.
- Simons, F E, Gu, X, Johnston, L M, Simons, K J. (2000b) Can epinephrine inhalations be substituted for epinephrine injection in children at risk for systemic anaphylaxis?Pediatrics. 106(5):1040–4 Simons, F E, Gu, X, Simons, K J. (2001a) Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 108:871–873.
- Simons, F E, Peterson, S, Black, C D. (2001b) Epinephrine dispensing for the out of hospital treatment of anaphylaxis in infants and children: a population based study. Ann Allergy Asthma Immunol. 86(6) 622–626.
- Simons, F E, Gu, X, Silver, N A, Simons, K J. (2002) Epipen Jr versus Epipen in young children weighing 15 – 30Kg at risk for anaphylaxis. J Allergy Clin Immunol. 109(1):171–175.
- Song, T T, Nelson, M R, Cheng, J H, Engler, R J, Chowdhury, B A. (2005) Adequacy of the epinephrine auto-injector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol. 94(5):539–542.
- Soreide, E, Buxrud, T, Harboe, S. (1988) Severe anaphylactic reactions outside hospital: aetiology, symptoms and treatment. Acta Anaesthesiol Scand. 32:339–342.
- Starks, B J, Sullivan, T J. (1986) Biphasic and protracted anaphylaxis. J Allergy Clin Immunol. 78:76–83.
- Stewart, A J, Ewan P J. (1996) The incidence, aetiology and management of anaphylaxis presenting to an accident & emergency department. Q J Med. 89:859–864.
- Uguz, A, Lack, G, Pumphrey, R, Ewan, P, Warner, J, Dick, J, Briggs, D, Clarke, S, Reading, D, Hourihane, J. (2005) Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy. 35(6):746–750.
- Unsworth, D J. (2001) Adrenaline syringes are vastly over prescribed. Archives of Disease in Childhood. 84(5):410–1
- Vickers, D W, Maynard, L, Ewan, P W. (1997) Management of children with potential anaphylactic reactions in the community: a training package and proposal for good practice. Clin Exp Allergy. 27(8):898–903.
- Yocum, M W, Butterfield, J H, Klein, J S, Volcheck, G W, Schroeder, D R, Silverstein, M D. (1999) Epidemiology of anaphylaxis in Olmsted County: a population based study. J Allergy Clin Immunol. 104:452–456.
- Zimmermann, B, et al. (2001) Food allergy: frequency of adrenaline administration, Can J Allergy Clin Immunol. 6:159–161.
- (No authors listed.) (2003) Injectable adrenaline for children. Drug Ther Bull. 41(3):21–24.
First Published June 2006
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-

