Screening for Diabetic Retinopathy: An Overview
Somdutt Prasad MS FRCS
Fellow in Diabetic Eye Disease
Arrowe Park Hospital
Upton, Wirral L49 5PE, UK
Probably the first written reference to diabetes is found in the Ebers Papyrus of ancient Egypt dating back to about 1550 BC. The disease knows no medical speciality boundaries. Diabetic retinopathy is a specific microvascular complication of both insulin dependant (type1) and non insulin dependant (type 2) diabetes. The prevalence of retinopathy is strongly linked to the duration of diabetes. After 20 years of diabetes nearly all patients with type 1 diabetes and over 60% of patients with type 2 diabetes have some degree of retinopathy. Upto a fifth of newly diagnosed diabetics have been found to have some retinopathy. A diabetic is 25 times more likely to go blind than a person in the general population.
Surveillance and treatment of diabetes-related complications should be part of routine care of all patients with diabetes. Treatment should try to ensure normoglycaemia as far as practicable. Intensive treatment designed to keep glucose levels close to normal has been shown to reduce the risk of developing long term complicatons including retinopathy and slow the progression of pre-existing retinopathy in Insulin-dependent diabetes . It is not unreasonable to assume a similar effect in non-insulin dependant diabetes. Risk factors (see text box) need to be assessed and managed appropriately. The natural history and screening recommendations for diabetic retinopathy, nephropathy, and neuropathy must be understood, since even advanced disease can be asymptomatic.
Risk factors for the development and progression of diabetic retinopathy
Duration of diabetes
- IDDM patients do not have retinopathy for first five years of their diabetes
- Years of diabetes before puberty does not count
Blood glucose control
- Tight control of blood glucose delays onset of retinopathy
- Tight control also slows the progression of established retinopathy
Renal involvement
- Proteinuria, elevated blood urea nitrogen and elevated blood creatinine are excellent predictors of the presence of retinopathy
- Microalbuminuria indicates a high risk of soon developing retinopathy
Systemic hypertension
Pregnancy- Women beginning a pregnancy without retinopathy have a 10% risk of developing background retinopathy
- Women with background retinopathy at the onset of pregnancy may show progression, fortunately, there is usually some regression after delivery.
- About 4% of pregnant women with background retinopathy will progress to proliferative retinopathy
- Those with untreated proliferative retinopathy at the onset frequently do poorly unless they are treated with panretinal photocoagulation
- Women with previously treated proliferative retinopathy usually do not worsen during the pregnancy.
The case for screening
The four WHO cardinal principles for screening are:
- The condition should be an important health problem with a recognisable presymptomatic state.
- An appropriate screening procedure which is acceptable both to the public and health care professionals should be available.
- Treatment for patients with recognisable disease should be safe, effective and universally agreed.
- The economic cost of early diagnosis and treatment should be considered in relation to total expenditure on health care, including the consequences for leaving the disease untreated.
Diabetic retinopathy conforms well to these principles. Diabetes is common affecting about 2% of the population. Of these 10-15% are type 1 diabetics and the remainder type 2 diabetics. Ten years after diagnosis the prevalence of retinopathy is 40-50% and after 20 years the prevalence is 90% . Diabetic retinopathy remains the commonest cause of blindness in the working age population in many countries including the UK and the USA. The importance of the problem has been reaffirmed by the St Vincent declaration, which has set a five-year target of reducing new blindness in Europe, by one-third .
Vision loss with diabetic retinopathy may result from several mechanisms. Macular oedema or ischaemia may impair central vision. The new blood vessels of proliferative retinopathy can bleed, leading to preretinal or vitreous haemorrhage. The fibrous tissue that accompanies the neovascular response can pull on the retina distorting it and this can lead to a tractional retinal detachment.
The Wisconsin Epidemiolgic Study of Diabetic Retinopathy (WESDR) has examined the epidemiolgic and demographic features of diabetic retinopathy and provided data on the natural course of the disease. Three major studies have contributed to understanding the natural course of diabetic retinopathy, the risk factors for visual loss and provided guidelines for the management of diabetic retinopathy. They are The Diabetic Retinopathy Study (DRS), The Early Treatment of Diabetic Retinopathy Study (ETDRS) and the Diabetic Retinopathy Vitrectomy Study (DRVS). The DRS established that panretinal photocoagulation could improve the prognosis of proliferative retinopathy . This was also confirmed by other large trials . The ETDRS and other studies established the benefit of focal laser photocoagulation in eyes with macular oedema. Laser photocoagulation in both these studies was beneficial in preventing further visual loss, but generally not beneficial in reversing already diminished acuity.
Several studies have reported the cost effectiveness of screening for retinopathy. They have established that screening for diabetic retinopathy saves vision at a relatively low cost, and this cost is many times less than the disability payments provided to people who go blind in the absence of a screening programme. In 1983 the annual cost of treating a diabetic at risk of blindness was estimated to be £387, compared with welfare benefits paid to a blind person of £3575 per annum . Similar results were reported in more recent American and European studies.
Facilities for laser treatment of diabetic retinopathy are widely available and are present in any hospital eye department in the UK. Screening services are organised locally and often achieve limited coverage .
The Screening Process
Many different modalities of screening are in use depending on local availability of facilities. These variables include number of available ophthalmologists, other trained healthcare professionals, equipment and resources available for screening. However whichever method is used should have sufficient sensitivity (>80%) and specificity (>80%) for a single modality screening process. Combining two modalities of screening (for example direct ophthalmoscopy in conjunction with retinal photography) provides excellent sensitivity, but increases the cost per case screened and is often only possible in a hospital based setting.
For any method of retinal examination used, visualisation is improved by using a dark room and dilating the pupil. A three-dimensional view greatly facilitates the evaluation of macular oedema. This can be achieved by retinal photography using stereo pairs or by indirect biomicroscopy on a slit lamp. The direct ophthalmoscope is limited in this respect as it offers a two-dimensional view. The small field of view offered by a direct ophthalmoscope also limits its usefulness as a screening tool. Thus training the personnel conducting screening in the use of indirect slitlamp biomicroscopy (for non-photographic methods) would increase the sensitivity of the system.
Recording and archiving of images have traditionally been done using 35-mm slides or Polaroid prints. The role of new technology in the form of digital computerised imaging offers the prospect of immediate high quality images that can be easily and quickly (often instantaneously) transferred from screening camera to a central reference centre. Storage and reproduction are inexpensive and quick using this medium.
To achieve near universal coverage the screening method has to be community based and the point of delivery within easy reach of the population. A central database, in the form of a diabetic register, which generates recalls is useful to ensure that people at risk do not "slip through the net" and is also valuable for auditing the effectiveness of the programme.
Different Screening Modalities
The task of screening would seem straight forward, but the present environment of care has led to diversity of views and practice and thus to much debate. Potential screeners for diabetic retinopathy are: -
- Ophthalmologists
- Diabetologists
- Junior hospital doctors
- Retinal photography services
- General practitioners
- Optometrists
- Combinations of all these
The implicit "gold standard" for identifying and grading retinopathy is a retinal examination using indirect biomicroscopy by a senior ophthalmologist or seven field stereoscopic photographs of each eye interpreted by experienced readers .
In some countries there are a sufficient number of ophthalmologists to undertake annual retinal examination for all diabetics. This is not possible in many areas. In 1994 there were an estimated 433 whole time consultant ophthalmologists in the UK, equating to one ophthalmologist for every 1100 diabetics . Diabetologists can provide eye screening as a part of the total package of diabetic care; they are experienced in this field and review the patient at regular intervals for their diabetic care. However a large proportion (40-60%) of diabetics are not seen by a diabetologist but are cared for by their general practitioners . Diabetologists may also have limited facilities, in that a dark room may not be available and retinal examination is mostly carried out using a direct ophthalmoscope.
Screening provided by ophthalmologist or diabetologists would have to be hospital based. This often involves long travelling distances, waiting times and extra visits to the hospital. Another hospital-based approach would be to utilise the services of junior hospital doctors. Typically a medical students experience is limited to, at best 10 hours of retinal observation and thus they tend to perform poorly as screeners for diabetic retinopathy . Their performance can be improved by appropriate training .
General practitioners are easily accessible to the patients and thus well placed to undertake screening in the community. However they may not get sufficient experience in the diagnosing and grading retinopathy. A practice with a list size of 7000 will have approximately 150 - 300 diabetics, thus they would see three to six diabetics per week of which one or two would have some retinopathy . They generally use direct ophthalmoloscopy and may lack dark room facilities. As a consequence eye screening provided by general practitioners often appears to be inadequate . Studies have shown that general practitioners are happy to refer patients to a variety of schemes that provide eye-screening .
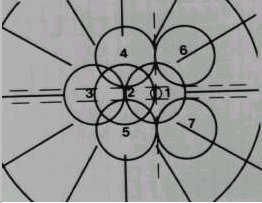
Many studies have shown that a mobile retinal photography service can provide an acceptable screening service . The "gold standard" consists of seven 30-degree fields using stereoscopic pairs (Figure 1). This needs two frames from each field to simulate a stereoscopic view; thus fourteen frames from each eye are needed. It has been shown that reducing this to two 45-degree fields per eye does not significantly alter the results whilst reducing the cost, complexity and the time spent . The photographs can be taken by a mobile unit with a camera and a technician and are later assessed by a trained reader or an ophthalmologist . This method is well suited to serve rural communities.
|
Figure 1. The seven standard fields. Each covers 30 degrees. |
Optometrists are generally accessible from the patient's home or workplace and in any case a large proportion of patients visit their optometrist on an annual or biannual basis. Optometrists have the facilities to measure visual acuity in a standardised way and a dark examination room. They are familiar with the use of mydriatics, which are needed to facilitate the examination. Facilities for slit lamp biomicroscopy are available at most practices and some optometrists are already performing indirect ophthalmoscopy on the slit lamp. Others can easily be trained to acquire this skill. Optometrists are therefore well suited to carry out screening for diabetic retinopathy in the community . It has been shown that with appropriate training they are able to detect diabetic retinopathy and make the correct decision regarding the need to refer the patient for secondary care. Optometrist based screening schemes are successfully in operation in various places .
Which one to choose?
The choice of screening modality to use in a given setting is dependent on local factors. The number of trained ophthalmologists available is the limiting factor in initiating an ophthalmologist based screening service in most countries. Because of this screening will have to be organised in an "ophthalmologist led" system rather than an "ophthalmologist based" system in most communities. The screening facilities have to be easily accessible if there is to be a high uptake and near universal coverage. General practitioners, optometrists and retinal photography can all provide easily accessible screening in the community.
General practitioners may not gain sufficient experience in the diagnosis and grading of diabetic retinopathy. This situation may be remedied by one partner in each practice taking on the role of a screener for retinopathy. He can then acquire the necessary skills and acquire the needed equipment for slit lamp indirect ophthalmoloscopy. However, this will mean setting up expensive equipment, which will only be used for a small number of patients a week. Some of the larger practices with an interested partner may find this to be a viable system. For many practices this will not be the prefferred modality. Therefore the choice of screening method will in most instances be either optometrist based or photography based.
Availability of resources and infrastructure together with local remunerative practices will dictate the choice between these. Some societies may find the cost of photography too high , but in other settings this may be the cheaper method of screening . The advent of digital computerised imaging has the potential to reduce the cost per patient and improve archiving and retrieval. Improvements in information technology have made instantaneous transfer of these images from screening centre to a referral centre possible. These may tilt the balance in favour of photographic techniques once these technologies are proven.
Future directions and issues
Issues that need to be addressed in order to improve the service are:
- What effect does frequency of encountering sight threatening diabetic retinopathy have on a primary screener's performance?
- The influence of mydriasis on screening performance. Should it be used uniformly rather than at the discretion of the screener?
- How do the patients view different screening modalities?
- The role of digital imaging and modern information technology.
- Increased training in and use of indirect ophthalmoscopic biomicroscopy by healthcare professionals delivering the screening service.
- In-built audit systems to help the systems evolve usefully.
Conclusion
Annual dilated fundus examination is desirable beginning 5 years after the diagnosis of type 1 diabetes mellitus and at the time of diagnosis of type 2 diabetes mellitus. The importance of this schedule is widely accepted. This objective continues to elude about half the patients with diabetes mellitus . A good start has been made in organising diabetic retinopathy screening, but in order to provide a sensitive, cost effective and easily accessible screening service which achieves universal coverage more schemes must be initiated, funded and audited. Key issues in the future will be the training of the screening personnel and meeting the cost of screening. Increasing use of indirect ophthalmoscopy on the slit lamp for retinal examination by non-ophthalmologists is desirable. Newer technologies including digital imaging may reduce the cost of screening. Diabetic registers will play an important role in ensuring attendance and continuing audit will ensure further improvements.
Thanks to Mr R P Phillips and Mr L Clearkin, consultant ophthalmologists at the Arrowe Park Hospital for going through the script and suggesting changes.
Bibliography
Aldington S.J., et al., (1995) Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia, . 38(4): p. 437-44.
Beer, G., (1996) The recognition and treatment of diabetes through the ages. Practical Diabetes International. 13(1): p. 33-34.
Bibby K., et al., (1992) Benefits of training junior physicians to detect diabetic retinopathy--the Glasgow experience. Journal of the Royal Society of Medicine, . 85(6): p. 326-8.
Brechner R.J., et al., (1993) Ophthalmic examination among adults with diagnosed diabetes mellitus [see comments]. JAMA. 270(14): p. 1714-8.
British Multicentre Study Group, (1977) Proliferative diabetic retinopathy: treatment with xenon-arc photocoagulation. Interim report of multicentre randomised controlled trial. British Medical Journal. 1(6063): p. 739-41.
British Multicentre Study Group,(1983) Photocoagulation for diabetic maculopathy. A randomized controlled clinical trial using the xenon arc. Diabetes, . 32(11): p. 1010-6.
The Diabetes Control and Complications Trial Research Group, (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 329(14): p. 977-86.
Diabetic Retinopathy Study Group,(1978). Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 85(1): p. 82-106.
The Diabetic Retinopathy Vitrectomy Study Research Group, (1985) Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Archives of Ophthalmology. 103(11): p. 1644-52.
Early Treatment of Diabetic Retinopathy Study Group,(1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study. Arch Ophthalmol, . 103(12): p. 1796-806.
Early Treatment of Diabetic Retinopathy Study Group,(1991) Grading diabetic retinopathy from stereoscopic color fundus photographs - An extension of the modified Airlie House classification. ETDRS reort number 10. Ophthalmology, . 98(5 Suppl): p. 786-806.
Finlay R., et al., (1991) Can general practitioners screen thier own patients for diabetic retinopathy? Health Trends, . 23(3): p. 104-105.
Gatling W., Howie A.J., and Hill R.D., (1995) An optical practice based diabetic eye screening programme. Diabetic Medicine, . 12(6): p. 531-6.
Greener M., (1996) Counting the cost of diabetes. Costs & Options in Diabetes, (10): p. 4-5.
Hammond C.J., et al.,(1996) Comparison between an ophthalmic optician and an ophthalmologist in screening for diabetic retinopathy. Eye, . 10(Pt 1): p. 107-12.
Harding S.P., et al., (1995) Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ, . 311(7013): p. 1131-5.
Harris A., et al., (1994)Commissioning diabetic eye screening by optometrists: a local initiative at the primary-secondary care interface. Journal of Medical Screening, . 1(1): p. 13-5. 31.
Harrison R.J., Wild J.M., and Hobley A.J.,(1988) Referral patterns to an ophthalmic outpatient clinic by general practitioners and ophthalmic opticians and the role of these professionals in screening for ocular disease. BMJ, . 297(6657): p. 1162-7.
Javitt J.C. and Aiello L.P.,(1996) Cost-effectiveness of detecting and treating diabetic retinopathy. Annals of Internal Medicine, . 124(1 Pt 2): p. 164-9.
Klein R., Klein B., and Moss S.,(1989) The Wisconsin Epidemiological Study of Diabetic Retinopathy: a review. Diabetes Metab Rev. 5: p. 5559-70.
Lairson D.R., et al., (1992) Cost-effectiveness of alternative methods for diabetic retinopathy screening . Diabetes Care. 15(10): p. 1369-77.
Mason J., Drummond M., and Woodward G., (1996) Optometrist screening for diabetic retinopathy: evidence and environment. Ophthalmic & Physiological Optics, . 16(4): p. 274-85.
Matz H., et al., (1996) Cost-benefit analysis of diabetic eye disease. Ophthalmologica, . 210(6): p. 348-53.
McCuish A.C.,(1993) Early detection and screening for diabetic retinopathy. Eye, . 7(2): p. 254-259.
Office of Health Economics,(1995) OHE Compendium of Health Statistics, OHE: London.
Singh K.J., et al., (1995) Screening for diabetic retinopathy. Annual retinal photography is not an option in India [letter; comment]. BMJ. 311(7014): p. 1230; discussion 1231.
de Sonnaville J.J., et al., (1996) Retinopathy screening in type 2 diabetes: reliability of wide angle fundus photography. Diabetic Medicine, . 13(5): p. 482-6.
Sullivan F.M., Stearn R., and MacCuish A.C., (1994) The role of general practitioners in diabetic eye care in Lanarkshire. Diabetic Medicine, . 11(6): p. 583-5.
Sussman E.J., Tsiaras W.G., and Soper K.A.,(1982) Diagnosis of diabetic eye disease. JAMA, . 247(23): p. 3231-4.
Taylor R., (1996) Practical community screening for diabetic retinopathy using the mobile retinal camera: report of a 12 centre study. British Diabetic Association Mobile Retinal Screening Group. Diabetic Medicine, . 13(11): p. 946-52.
WHO / International Diabetic Federation, (1990) Diabetes Care and Research in Europe; The Saint Vincent Declaration, in Diabetic Medicine. p. 360.
Williams R., (1994) Diabetes in Medicine, in Health Care Needs Assesment: The Epidemiologically Based Needs Assesment Reviews, A. Stevens and J. Rafferty, Editors, Radcliffe Medical Press: Oxford.
Copyright © Priory Lodge Education Limited, 1997.
Version 1.0 first published 8/6/97
This version published 23/03/99
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages copyright ©Priory Lodge Education Ltd 1994-