Browse through our Journals...
Self-Reported Impressions of Insulin Detemir Among Patients with Type 2 Diabetes: Insulin-Naïve vs. Prior Insulin Users
Donna L Kerney, PhD1, Stephen Brunton, MD2
1InfoMedics, Inc., Woburn, MA; 2Cabarrus Family Medicine Residency, Concord, NC
Corresponding Author:
Donna L. Kerney
InfoMedics, Inc.
12 Gill Street, Suite 2600
Woburn, MA 01801
SUMMARY
The purpose of this research was to assess differences in perceptions of insulin detemir among patients with type 2 diabetes naïve to insulin vs. prior insulin users. Physicians identified patients >18 years appropriate for treatment with insulin detemir. Patients voluntarily provided consent and responded to surveys prior to and 30 and 60 days following insulin detemir initiation. Patients reported their perceptions of insulin detemir using an automated telephone system. Average ratings on scales of 0 to 10 (10=positive response) were compared between insulin-naïve and prior insulin users. A total of 404 adults completed surveys. Average age was 61; 62% female. Sixty-three percent used insulin prior to insulin detemir. Ease in judging blood sugar levels with insulin detemir was higher among insulin-naïve compared with prior users after 35 (average 7.2 vs. 6.4) and 71 days (7.6 vs. 6.6). Ease of keeping good blood sugar control with insulin detemir was higher among insulin-naïve than prior users after 35 (7.3 vs. 6.4) and 71 days (7.7 vs. 6.6). Satisfaction with insulin detemir was higher among naïve patients after 35 (8.5 vs. 7.6) and 71 days (8.6 vs. 7.6). All reported differences were statistically significant (p<0.01).
Insulin detemir is well-received as treatment for type 2 diabetes, particularly among insulin-naïve patients.
Keywords: insulin detemir; type 2 diabetes; patient-reported outcomes; insulin
INTRODUCTION
Approximately 7-9% of the US population has diabetes (ADA, 2007; NCHS, 2007), with 90-95% of cases being type 2. In 2005, 1.5 million new cases were diagnosed. (ADA, 2007; NCHS, 2007) Within the last decade, increased emphasis has been placed on the need for adequate glycemic control to prevent the serious and long-term complications that can result from diabetes. Poorly controlled diabetes has been associated with eye, kidney and nerve diseases. (Diabetes Control and Complications Trial Research Group, 1993) The Diabetes Control and Complications Trial (DCCT) showed that keeping blood glucose at normal or near normal levels slows the onset and progression of these diseases. (DCCT, 1993) Estimates from 2002 indicate that in the United States, diabetes costs $132 billion, including $92 billion in direct medical costs and $40 billion in disability, lost productivity and premature death. (CDC, 2005) Achieving and maintaining glycemic control is paramount to management of diabetes and reducing the impact of disease on patients. The importance of achieving glycemic control is highlighted in that for every percentage point decrease in A1c values, the risk of microvascular diseases (e.g., eye, kidney or nerve diseases) is reduced by 40%. (CDC, 2005)
The American Diabetes Association (ADA) has stated a goal A1c of less than 7.0%, or “as close to normal as possible (<6%) without significant hypoglycemia.” The American College of Endocrinology and the American Association of Clinical Endocrinologists (ACE/AACE) (ACE/AACE, 2007) have developed a “Road Map for the Prevention and Treatment of Type 2 Diabetes” to assist the medical community in treating patients with type 2 diabetes to reach their glycemic control recommendation for an A1c level of less than or equal to 6.5%. This level applies to both treatment naïve and treated patients. (ACE/AACE, 2007) The Road Map recommends lifestyle modifications followed by oral antidiabetic medications (OADs) as mono or combination therapy, followed by insulin. If an A1c level exceeds 10.0%, ACE/AACE recommend insulin therapy as initial pharmacologic treatment. Monitoring and medication titration every few months are suggested, with increases in intensity or add-on therapies being applied as needed to achieve goal A1c for all patients. (ACE/AACE, 2007) Earlier initiation of treatment with insulin is also recommended for patients with special circumstances. (ACE/AACE, 2007)
Estimates from the National Institutes of Health indicate that 16% of adults diagnosed with type 1 or type 2 diabetes use insulin only, 57% use oral medication only, 12% use both and 15% use neither. (NIH, 2007) When used correctly, insulin therapy is one of the most effective treatment options for improving glycemic control. However, hypoglycemia and weight gain are potential side effects associated with insulin use. In an effort to address the disadvantages of human insulin preparations, insulin analogs have been developed to more closely mimic physiologic insulin secretion while minimizing the risk of hypoglycemia. (Kamal et al, 2006) Insulin detemir is a long-acting basal insulin analog with pharmacokinetic properties that allow the delivery of insulin with prolonged and consistent action. (Haak et al, 2005; Raslova et al, 2004) Based on data from randomized controlled trials involving patients with type 2 diabetes, this insulin analog has been shown to help patients achieve glycemic control (Haak et al, 2005; Raslova et al, 2004; Hermansen et al, 2006; Rosenstock et al, 2006; Klein et al, 2007; Raslova et al, 2004; Philis-Tsimikas et al, 2006) with a lower risk of hypoglycemia and/or less weight gain when compared with NPH insulin and insulin glargine.
Hermansen and colleagues conducted a randomized, parallel, treat-to-target trial of 476 insulin-naïve patients in which subjects were randomized to receive insulin detemir or NPH insulin for 26 weeks. The authors reported a significantly lower risk of hypoglycemia with insulin detemir compared with NPH insulin (p<0.001). They also noted a lower average weight gain among those using insulin detemir (p<0.001). (Hermansen et al, 2006) Improved glycemic control with insulin detemir was also evident in two randomized trials that examined the efficacy and safety of a basal-bolus regimen that included insulin detemir. (Haak et al, 2005; Raslova et al 2004) While achievement of glycemic control was similar among those receiving insulin detemir vs. NPH insulin (Haak et al, 2005), patients using insulin detemir experienced significantly less weight gain and lower within-subject variability in fasting self-monitored blood glucose. (Haak et al, 2005; Raslova et al 2004) Results from the Raslova et al. study also showed a significantly lower risk of nocturnal hypoglycemia (p=0.04) in the insulin detemir group. (Raslova et al, 2004) In their randomized trial, Philis-Tsimikas and colleagues compared the effectiveness and tolerability of insulin detemir versus NPH in poorly controlled patients with type 2 diabetes using at least one OAD and assessed differences in morning versus evening administration. (Philis-Tsimikas et al, 2006) The authors concluded that insulin detemir can be used in the morning or evening to improve glycemic control in this population. (Philis-Tsimikas et al, 2006)
While clinical studies have documented the efficacy of insulin detemir in achieving glycemic control among patients with type 2 diabetes, the impressions of patients using the medication outside the constraints of a clinical trial are less well understood, as are any differences in the impressions of patients using insulin for the first time vs. experienced insulin users. Patient perceptions of their therapy can have an impact on their compliance and subsequently their treatment outcome (Chaufan et al, 2000; Oparil et al, 1998; Cushman et al, 2000; Dezii, 2000), i.e., glycemic control. Non-adherence to medication results in increased morbidity and mortality for patients as well as increased costs to the health care system and general economy, including $100 billion (Dezii, 2000) in direct medical costs, hospitalizations, lower productivity and premature death.
Psychological barriers to insulin use can be significant detriments to treatment initiation and maintenance. These perceptions, and potential barriers, may differ for insulin naïve patients relative to those of experienced insulin users. Negative perceptions among patients just starting treatment could delay the use of needed therapy. These patients may need more encouragement or more explicit expectations set by their providers. Experienced insulin users have an inherent comparison of their current treatment with their prior therapy. Negative or positive perceptions of prior therapy may lead these patients to assume negative or positive perceptions of a new therapy. While these patients have accepted the use of insulin, they may still harbor some resistance to continued use or use of another insulin if their experience has been less than optimal. For any medication, such as insulin detemir, patient information can be very valuable for physicians. Such information could help physicians to assess patients’ perceptions and tailor their management of patients in terms of compliance, accordingly. Data from patients using the medication in the course of everyday living can help physicians more rapidly gain experience and knowledge about the treatment. The perceptions of patients who are naïve to insulin vs. experienced insulin users can be different and therefore the objective of this analysis was to assess the impressions of insulin detemir between these two groups.
METHODS
Data for this survey study were obtained from patient surveys that included questions related to patients’ experiences with insulin detemir for treatment of diabetes. A total of 15,322 physicians from the United States participated in this study that spanned January – September 2006. Physicians identified patients from their practices who were at least 18 years of age, diagnosed with diabetes, and appropriate candidates for treatment with insulin detemir. Eligible patients received study materials, and those who wished to participate voluntarily enrolled by calling a toll-free number and providing consent after listening to how the information will be used and pressing the appropriate key on the telephone keypad to confirm agreement to participate or decline. Patients who decline are thanked for calling and disconnected. Those who agree are transferred into the automated survey system. The survey study involved patients completing three automated telephone surveys, before use of insulin detemir, at 30 days and at 60 days post-medication initiation. Clinical personnel on the study team designed the surveys to include questions to measure important patient perceptions regarding use of insulin detemir, including ease of judging and maintaining good blood sugar control, confidence in avoiding symptoms of hypoglycemia and low blood sugar at night and medication satisfaction.
Patients used touch-tone telephones to complete the surveys. Responses from all surveys were recorded in a secure database. In order to complete each follow-up survey, patients were required to enter selected, personal information to verify their record and permit them to complete the surveys. Patients received reminders throughout the process to alert them to take their next survey. Physicians did not receive any compensation or incentive for their participation. In this survey study from a convenience population, data reflect patients’ own perceptions and impressions, not professional clinical evaluations.
No individual patient identifiable information is included in the aggregate results presented in this paper. In addition, the medication was prescribed according to each physician’s discretion and normal course of treatment, and therefore was deemed to not require the need for IRB review. No formal or informal protocol for medication use was included in this study. Each physician prescribed the medication for each patient on an individual basis.
The baseline survey included questions about type of diabetes, use of prescription pills to control diabetes prior to insulin detemir, use of insulin, and satisfaction with prior diabetes medications. In subsequent surveys, completed approximately 30 and 60 days after treatment initiation, patients rated the ease of judging blood sugar levels and the ease of keeping good blood sugar control with insulin detemir relative to their prior treatment using a scale of 0 to 10 (0=much more difficult;10=very much easier). Prior insulin users rated their relative confidence in avoiding symptoms of hypoglycemia and low blood sugar at night with insulin detemir vs. their prior insulin on a 0 to 10 scale (0=much less confident; 10=much more confident). Data were collected on patients’ satisfaction with their prior medication and with insulin detemir. Again, patients used a scale from 0 (not at all satisfied) to 10 (very satisfied) to rate their satisfaction with medication. On the 60-day survey, patients who had used insulin prior to insulin detemir were asked to report whether they lost weight, gained weight or had no change in weight since starting insulin detemir. Those who had a weight change reported the number of pounds gained or lost.
The surveys were available to patients with type 1 or type 2 diabetes, however the focus of this paper is on type 2 diabetes. Survey responses from all patients with type 2 diabetes who completed the three surveys were summarized and reported as frequency distributions or mean values, as appropriate. Responses of patients who were naïve to insulin vs. those who used insulin previously were compared. T-tests or Chi-Square analyses were conducted as appropriate to determine statistical significance in outcomes between the groups.
Analyses were conducted using SPSS v. 11.5.0 statistical software.
RESULTS
A total of 2,908 patients voluntarily enrolled in the survey study. Of these, 20% (n=586) completed all surveys; and 69% (n=404) of those patients reported having a diagnosis of type 2 diabetes. Only data collected for these 404 patients with type 2 diabetes are reported in this paper. The average time between the baseline and first follow-up survey was 35 days; between baseline and second follow-up was 71 days. Insulin naïve patients represent 37% (n=151) of patients. Table 1 contains baseline characteristics of the participants.
Patient Perceptions of Insulin Detemir: Insulin Naïve vs. Prior Insulin Users
Overall, patients in both groups rated ease of judging blood sugar levels with insulin detemir as easier than judging blood sugar levels with their prior therapy. After an average of 35 days with insulin detemir, patients naïve to insulin rated the ease of judging blood sugar levels an average of 7.2 out of 10 (10=much easier) vs. 6.4 among the prior users (p<0.01) (Figure 1). Similar results were reported an average of 71 days following treatment initiation, with the insulin naïve patients rating ease of judging blood sugar levels higher than prior users (7.6 vs. 6.6; p<0.01).
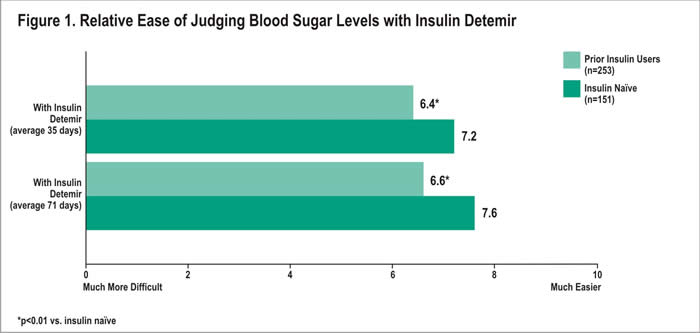
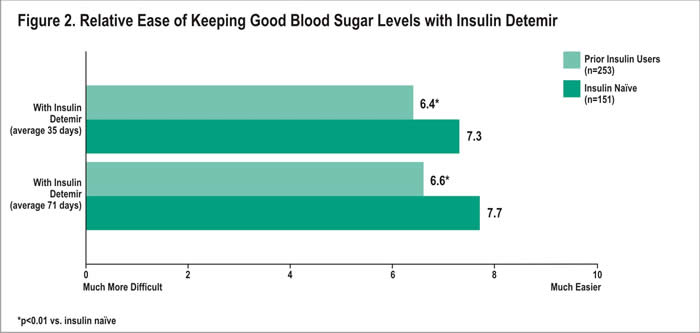
Similarly, patients from both groups felt that with insulin detemir, it was easier to keep good blood sugar control compared with their prior treatment. Ease of keeping good blood sugar control with insulin detemir averaged 7.3 among insulin naïve patients, compared with 6.4 among prior insulin users after 35 days with treatment (p<0.01) (Figure 2). After an average of 71 days, the mean rating was again higher among the naïve patients compared with the prior users (7.7 vs. 6.6, respectively; p<0.01).
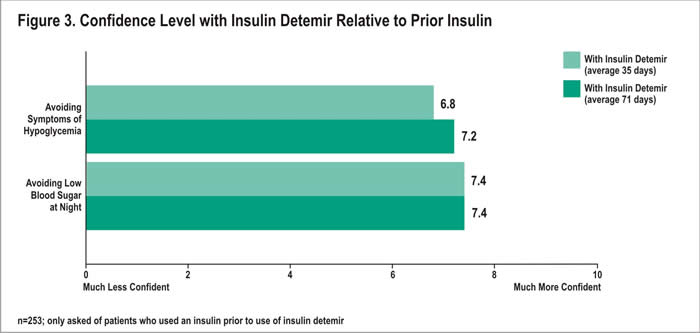
Relative Confidence in Avoiding Symptoms of Hypoglycemia: Prior Insulin Users
Patients who had used insulin prior to insulin detemir were asked about their relative confidence in avoiding symptoms of hypoglycemia and avoiding symptoms at night. Overall, prior insulin users felt more confident in avoiding symptoms of hypoglycemia with insulin detemir than they did with their prior insulin (Figure 3). Mean confidence ratings of 6.8 and 7.2 were reported after 35 and 71 days, respectively. Forty-seven percent of prior insulin users rated their relative confidence at 8 or higher (10=much more confident) after 35 days; 53% 71 days following treatment initiation. Conversely, after 35 days with insulin detemir, 6% of prior insulin users were less confident (rating at 2 or less; 0=much less confident) about avoiding symptoms of hypoglycemia with insulin detemir than they were with their prior insulin. After an average of 71 days with insulin detemir, only 2% reported low confidence.
In addition, prior insulin users rated their confidence in avoiding low blood sugar at night with insulin detemir compared with their prior insulin. An average of 35 and 71 days following treatment with insulin detemir, patients rated their relative confidence with insulin detemir at 7.4 out of 10 (Figure 3). After 35 days, 58% reported high confidence, with a rating of 8 or higher; after 71 days, 60% reporting these ratings. In contrast, at 35 days, only 3% reported a rating of 2 or less (0=much less confident) for their confidence. After 71 days, 4% reported these ratings.
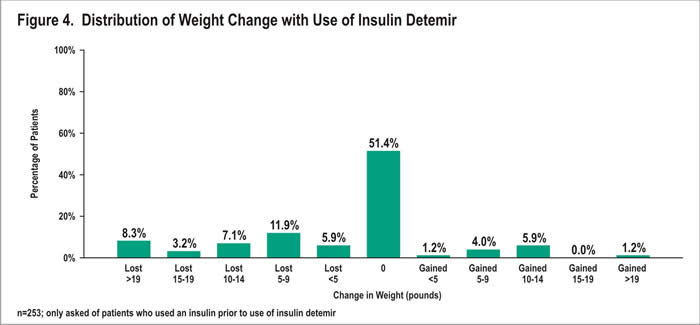
Change in Weight: Prior Insulin Users
Among prior insulin users, most (51%) reported no change in weight since starting insulin detemir (Figure 4). Twelve percent gained weight; 36% lost weight. Those who gained weight reported an average gain of 9.9 pounds, with a range of 3-40 pounds and a median of 10 pounds. Patients who lost weight lost an average of 13.3 pounds, with a range of 2-60 pounds lost and a median of 10 pounds lost. Eight percent lost more than 19 pounds.
Medication Satisfaction: Insulin Naïve vs. Prior Insulin Users
Overall, patients rated satisfaction with insulin detemir at over 7.5 out of 10. Patients naïve to insulin had a mean satisfaction rating of 8.5 out of 10 (10 = very satisfied) after 35 days with insulin detemir vs. 7.6 among prior insulin users (p<0.01). Satisfaction remained high after 71 days, with mean ratings of 8.6 and 7.6 for insulin naïve and prior insulin users, respectively (p<0.01).
DISCUSSION
Clinical trial data support the effectiveness of insulin detemir as treatment for diabetes. (Haak et al, 2005; Raslova et al, 2004; Hermansen et al, 2006; Rosenstock et al, 2006; Klein et al, 2007; Raslova et al, 2004; Home et al, 2006) Clinical efficacy is paramount to a positive response, but patients’ perceptions about treatment cannot be overlooked. Ultimately, patients must decide to follow the treatment regimens prescribed by their physicians, and their attitudes and feelings about how a medication is performing for them are important to consider.
While overall, patients reported it was easier to judge and keep good blood sugar control with insulin detemir compared to their prior therapy, insulin naïve patients tended to report higher ratings compared with the prior insulin users. The higher average satisfaction among insulin naïve vs. prior insulin users is consistent with the former’s higher average ratings for ease of judging and keeping good blood sugar control. Prior insulin users may inherently be making comparisons to their prior insulin rather than rating their experience with insulin detemir independently. In addition, for prior insulin users, insulin detemir is at least the second insulin they have tried. They may tend to have lower ratings because they have tried other insulins that were unsuccessful to some extent and may be more prone to give conservative responses regarding the impact of the new insulin. Results from this patient survey study also suggest that experienced insulin patients are more confident using insulin detemir to control blood sugar levels and avoid symptoms of hypoglycemia than they were with their prior insulin. This finding complements clinical studies that have shown a lower risk of nocturnal hypoglycemia with insulin detemir compared to other basal insulin preparations. (Haak et al, 2005; Raslova et al, 2004; Hermansen et al, 2006) The lower weight gain reported by patients through their survey responses has also been demonstrated in randomized clinical trials. (Haak et al, 2005; Raslova et al, 2004; Hermansen et al, 2006; Rosenstock et al, 2006) In addition, data from PREDICTIVE (Predictable Results and Experience in Diabetes through Intensification and Control to Target: An International Variability Evaluation), a large, multi-center, prospective, observational study to assess safety and efficacy of insulin detemir, support results from clinical trials that suggest insulin detemir improves glycemic control without weight gain and reduces nocturnal episodes of hypoglycemia. (Dornhorst et al, 2007; Meneghini et al, 2007)
This real-world survey has several limitations. When participation is voluntary, selection bias may be present. While physicians identified eligible patients from their practices, patients themselves chose whether to participate. Selection bias may occur if physicians choose certain patients to inform about the program, and if patients who ultimately choose to participate differ from those who choose not to participate in the surveys. No data were available to determine the extent to which selection bias may have impacted the patient sample, as no information is available about those patients who chose not to participate. Results therefore may not be generalizable to or indicative of the experiences of all patients using insulin detemir to treat diabetes. In addition, patients were not required to follow a protocol when using the medication. Rather, physicians directed the prescribing for each patient as part of their normal course of clinical practice. The survey was designed as a real world assessment of patients’ self-reported perceptions of insulin detemir. No control group was used. None of the information provided by patients, including their adherence to therapy, was validated or confirmed through objective measures (e.g., clinical testing or retrospective claims data review). All responses are subjective and based solely on patients’ perceptions. Patients’ determinations of their ability to judge control were self-reported perceptions, not based on clinically defined parameters. The extent to which patients may have given socially desirable answers is unknown. Lastly, while the authors acknowledge no formal validation of the survey instruments, the questions were developed by clinical personnel with knowledge of diabetes treatments and have strong face validity. Patient-reported outcomes questionnaires are frequently used to gather data about medical treatments and therapies from patients’ perspectives. Despite the limitations, the authors contend that the survey met the objectives of collecting valuable patient-reported impressions about insulin detemir from patients with type 2 diabetes and comparing the responses of those new to insulin vs. experienced insulin users.
The findings from the patient surveys demonstrate that patients overall have very positive impressions of insulin detemir as treatment for type 2 diabetes. Insulin naïve patients had higher ratings than prior insulin users for ease of judging blood sugar and keeping good blood sugar levels. An additional study to assess patients’ impressions after longer use of insulin detemir may be of value. Retrospective database studies that examine other factors (e.g., comorbidities, concurrent medications) that may impact differences in perceptions among insulin naïve vs. prior insulin users might be complementary to the self-reported patient data collected in these surveys. Also, further examination into the extent to which the individual patient data can help to guide physicians’ decision-making and monitoring of patients’ perceptions of treatment would be of interest.
CONFLICT OF INTEREST STATEMENT
Dr. Brunton is a consultant for Novo Nordisk, Amylin, Pfizer, Abbott.
Dr. Kerney is an employee of the company contracted by Novo Nordisk to implement the surveys.
ACKNOWLEDGEMENTS:
The authors acknowledge Novo Nordisk for funding this project. The authors also acknowledge Rebecca Shaffer for her editorial assistance in reviewing this manuscript.
REFERENCES
1. American College Endocrinology/American Association of Clinical Endocrinologists Diabetes Road Map Task Force. Road map for the prevention and treatment of type 2 diabetes. Available at: http://www.aace.com/meetings/consensus/odimplementation/roadmap.pdf. Accessed January 2007.
2. American Diabetes Association. Total prevalence of diabetes and pre-diabetes. Available at: http://www.diabetes.org/diabetes-statistics/prevalence.jsp. Accessed January 2007.
3. Centers for Disease Control. National diabetes factsheet. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. Accessed January 2007.
4. Chaufan C. (2000) Patient compliance: In search of the real question in diabetes care. American Family Physician. 61, 644–647.
5. Cushman WC, Reda D J, Perry H M, et al., for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. (2000) Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States. Archives of Internal Medicine. 160, 825–831.
6. Dezii C M. (2000) Medication noncompliance: What is the problem? Managed Care. 9 (Suppl 9), 7–12.
7. Dornhorst A, Luddeke H-J, Sreenan S, et al. (2007) Safety and efficacy of insulin detemir in clinical practice: 14-week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVE European cohort. International Journal of Clinical Practice. 61, 523-528.
8. Haak T, Tiengo A, Draeger E, et al. (2005) Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obesity and Metabolism. 7, 56-64.
9. Hermansen K, Davies M, Derezinski T, et al. (2006) A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetes Care. 29, 1269-1274.
10. Home P, Kurtzhals P. (2006) Insulin detemir: from concept to clinical experience. Expert Opinion on Pharmacotherapy. 7, 325-343.
11. Kamal A D, Dixon A N, Bain S C. (2006) Safety and side effects of the insulin analogues. Expert Opinion on Drug Safety. 5, 131-143.
12. Klein O, Lynge J, Endahl L, et al. (2007) Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obesity and Metabolism. 9, 290-299.
13. Meneghini LF, Rosenberg K H, Koenen C, et al. (2007) Insulin detemir improves glycemic control with less hypoglycemia and no weight gain in patients with type 2 diabetes who were insulin naïve or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obesity and Metabolism. 9, 418-427.
14. National Center for Health Statistics. FASTSTATS A to Z. Diabetes. Available at: http://www.cdc.gov/nchs/fastats/diabetes.htm. Accessed January 2007.
15. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics. http://diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed January 2007.
16. Oparil S, Calhoun D A. (1998) Managing the patient with hard-to-control hypertension. American Family Physician. 57, 1007–1014, 1019–1020.
17. Philis-Tsimikas A, Charpentier G, Clause P, et al. (2006) Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clinical Therapeutics. 28, 1569-1581.
18. Raslova K, Bogoev M, Raz I, et al. (2004) Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Research and Clinical Practice. 4, 193-201.
19. Raslova K, Bogoev M, Raz I, et al. (2004) Corrigendum to: Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Research and Clinical Practice. 66, 193-201.
20. Rosenstock J, Davies M, Home P D, et al. (2006) Insulin detemir added to oral anti-diabetic drugs in type 2 diabetes provides glycemic control comparable to insulin glargine with less weight gain. Diabetes. 55, A132.
21. The Diabetes Control and Complications Trial Research Group. (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 329, 977-986.
First Published February 2008
Copyright Priory Lodge Education Limited 2008
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


