Development
of Matrix and coated units for
pH- independent release
of a weakly basic drug.
Munish Dhiman, S. S. Poddar, A. Shajahan
Department of Pharmaceutics,
Prin. K. M. Kundnani College
Dr. R. G. Thadani Marg, Worli Sea
Mumbai – 400 018 (India
Address for
correspondence:
Dr. S. S. Poddar
Department of Pharmaceutics,
Prin. K. M. Kundnani College
Dr. R. G. Thadani Marg,
Worli Sea India
E-mail: ssp306@rediffmail.com
mkd244@yahoo.co.in
Abstract
Weakly basic drugs have pH-dependent solubility. The resulting release
from tablets decreases with increasing pH-mileu of gastrointestinal tract.
The aim of this work was to overcome this problem by utilizing Succinic acid
as pH adjuster and to achieve
pH-independent release from matrix and coated tablets.
Eudragit-RSPO was used as matrix former and Eudragit-RSPO & RLPO mixture was employed for coating of tablets. Drug release from tablets was studied in pH 1.2 and 6.8 buffers. Effect of addition of Succinic acid on drug release and drug : Succinic acid ratio on drug release was studied. Acid base indicator methyl red was introduced into tablets to visually monitor the maintenance of acidic microenvironment inside the tablets. The release of Verapamil hydrochloride from Eudragit-RLPO and RSPO coated tablets was found to be constant and more pH-independent than matrix tablets containing Eudragit RSPO.
KEY WORDS
Weakly basic drug, pH-independent release, microenvironment.
Introduction
There
are certain challenges associated with the formulation of drugs with
pH-dependent solubility affecting the release from dosage and hence absorption
[Sanghvi, N. et al, 1994]. Verapamil HCl, a calcium channel blocker, exhibits
very high solubility in the gastric pH which falls drastically in the intestinal
pH [Florey, 1988; Streubel, A. et al, 2000]. It is imperative that a controlled
release oral formulation should have uniform release pattern at different
sites of G.I.T. over the period of dosing [Ritchel, 1989].
The aim of this work was to evaluate the in vitro performance of SR matrix and coated dosage formulations of Verapamil HCl for pH- independent release utilizing Succinic acid as pH-adjuster for the maintenance of constant acidic micro-environment inside the tablet core.
In accordance with the data obtained on solubility, different SR oral dosage forms of Verapamil HCl containing Succinic acid were developed in order to obtain near constant and complete release of the drug in the G.I.T. For this purpose the drug release was controlled by fabricating matrix as well as coated tablets. For the former, Eudragit-RSPO was used and for the latter, the drug containing core units were coated with a mixture of Eudragit-RSPO and Eudragit-RLPO.
Materials and Methods
Following materials were used as received:
Verapamil hydrochloride [Nicholas Piramal India Ltd., Mumbai]
Microcrystalline Cellulose (Vivacel-101) [J.
Eudragit- RSPO [Degussa Pharma Polymers,
Eudragit- RLPO [Degussa Pharma Polymers,
Polyethylene glycol – 4000 (PEG-4000) [SD Fine Chemicals, Mumbai]
Succinic acid [SD Fine Chemicals, Mumbai]
Methyl red [ Qualigens, Mumbai]
Magnesium stearate, I.P. [Research Lab Chemical Corporation, Mumbai]
Determination of Solubility of drug and Succinic acid
Solubility of Verapamil HCl has been sited in literature. At 370C, in pH 1.2, it is >150 mg/ml and at pH 6.8 it is 2.71 mg/ml. [Florey, 1988; Streubel, A. et al, 2000] The solubility of Succinic acid was determined gravimetrically at 370C, which was found to be 65 mg/ml at pH 1.2 and 107 mg/ml at pH 6.8.
Preparation of Matrix Tablets
Verapamil HCl, Succinic acid and MCC were dry blended and granulated using Eudragit-RSPO solution in IPA and water mixture (8.5 : 1.5). The wet mass was passed through a#16 sieve and the granular material was air dried for 1 hour followed by drying in hot air oven at 450C for 2 hrs. The dried mass was treated through #16 sieve superimposed on #20 / #40. The #16 / #20 and #20 / #40 fractions were mixed in 9:1 ratio. After lubrication with magnesium stearate compression was carried out using 10 mm flat faced circular punches on a 10 station rotary compression machine (Jaguar, Mumbai). Hardness of tablets was maintained at 6-7 kg / cm2.
Preparation of Coated Tablets
Coated tablets consisted of core and coat. Core of tablets contained drug, MCC and Succinic acid were compressed using 10 mm standard concave punches on a 10 station rotary compression machine. Hardness of tablets was kept at 5-6 kg/cm2.
These tablets were then coated with release retardant polymers by pan coating technique. The composition of the core was as described in table 2.
Coating Solution : Coating solution consisted of release retardant polymers Eudragit-RSPO, Eudragit-RLPO, Plasticizer PEG-4000, IPA and acetone.
Eudragit RSPO – 9 g
Eudragit RLPO – 1 g
PEG 4000 – 1.1 g
Solvent system – 100 ml
Solvent system consisted of Isopropylalcohol : Acetone (1:1)
Drug Release studies from Tablets
The in vitro drug release studies were carried out using USP type 2 dissolution apparatus, at 37± 0.50C and paddle speed of 50 rpm in buffer of pH 1.2 and pH 6.8 for first 2 hours and next 8 hours respectively. The aliquots were withdrawn at definite intervals of time. The drug content was determined spectrophotometrically at 278 nm [USP 25 / NF 20].
Inclusion of Indicator
To confirm the concept of creation and maintenance of adequate acidic microenvironment, pH- indicator methyl red (0.2% w/w) was introduced in the matrix tablets as well as core of coated formulations to visually monitor the pH within the tablets during drug release. This indicator is red in acidic pH and yellow in pH values > 5.8 [Streubel, A. et al 2000]. Below this pH value Verapamil is freely soluble. In case of matrix tablets sections were taken with blade so as to enable monitoring the color change clearly through the tablet body.
Results and Discussion
A) Matrix Tablets
1.
Effect of inclusion of Succinic acid on release
Fig. 1 and 2 reveal the dissolution profiles of matrix tablets without and with Succinic acid as pH-adjuster. The release rate falls in pH 6.8 from matrix tablets without Succinic acid. In tablets, containing Succinic acid the dissolution rate of the drug was high even in the elevated pH corresponding to intestine, which was due to maintenance of constant acidic pH inside the tablet core.
2.
Effect of ratio of Drug to Succinic acid
Various proportions of Succinic acid and drug were tried in matrix formulations. Succinic acid : drug ratio of 0.8:1 in the case of 15% polymer gave 100% drug release in 10 hours. Increasing this ratio above 1:1 lead to compression difficulties, while with ratios below this, sufficiently pH-independent release profile could not be achieved. This was evident from release at pH 6.8 which showed a fall in release rate (Fig. 2). There was slight fall in release rate from formulation containing Succinic acid : drug ratio of 1:1 compared to 0.8:1. This could be attributed to the reason that as the acid content was raised, there was reduction in MCC content (Table 1) which had a role to play in swelling of the tablet and thus in drug release.
B) Coated Tablets
1.
Effects of addition of organic acid on release
Fig. 3 reveals the dissolution profile of coated tablets containing Succinic acid. The polymer coat thickness was optimized at 5.8% weight rise with Eudragit RSPO and RLPO in 9:1 ratio. At this coat thickness it was evident that successful drug release retardation and desirably extended release could be achieved in pH 6.8, also no burst release was observed in pH 1.2.
2.
Effect of ratio of Succinic acid and Drug on release
In coated tablet formulation with Succinic acid : drug proportion of 0.6:1, 100% drug release was achieved in 10 hours. Decreasing this proportion to 0.4:1, sufficiently pH- independent profiles could not be achieved. Increasing the proportion above 0.8:1 resulted in coating difficulties (twinning and sticking) and there was slight decrease in release rate. This could be due to the decrease in MCC content as the Succinic acid concentration was raised. (Fig.3)
C) Indicator studies
In case of matrix tablets, color change related to the presence of Succinic acid could not be fully judged from surface. This may be due to the faster leaching of indicator as well as Succinic acid from the tablet surface. But the transverse sections of matrix tablets revealed the color change from red to pink to yellow as the dissolution progressed.
In coated tablets absence of development of yellow color was noted. Although in these tablets a color change i.e. red to pink was observed. This may be due to fall in strength of indicator due to leaching.
Inspite
of this color change it was indicative that there was sufficient Succinic
acid present inside the core and hence maintaining the solubility of the drug
high even after transition of media from pH 1.2 to 6.8, as dissolution progressed.
Conclusion :
From
the drug release profiles of matrix and coated tablets it was found that coated
tablets produced better pH independent release profiles than matrix tablets.
This indicated that there was better maintenance of acidic microenvironment
inside the coated tablets than matrix tablets. Support for maintenance of
microenvironment of low pH inside the tablet was also provided through the
use of indicator. Thus, it could be
concluded that Eudragit coated tablets with microenvironment provided by Succinic
acid demonstrated better
pH-independent release profile than matrix versions.
ACKNOWLEDGEMENTS :
The authors are thankful to Mr. Dilip Swain of Degussa Pharma Polymers, Mumbai for providing gift samples of polymers.
Table 1 : Formulation of Matrix Tablets
Components |
Composition (mg) |
|||
M1 |
M2 |
M3 |
M4 |
|
|
Verapamil HCl |
120 |
120 |
120 |
120 |
|
MCC |
286 |
214 |
190 |
166 |
|
Eudragit - RSPO |
72 |
72 |
72 |
72 |
|
Succinic acid |
- |
72 |
96 |
120 |
|
Magnesium Stearate |
2 |
2 |
2 |
2 |
Table 2 : Formulation of Core of Tablets
Components |
Core Composition (mg) |
||
|
C1 |
C2 |
C3 |
|
|
Verapamil HCl |
120 |
120 |
120 |
|
MCC |
310 |
286 |
262 |
|
Succinic acid |
48 |
72 |
96 |
|
Magnesium stearate |
2 |
2 |
2 |
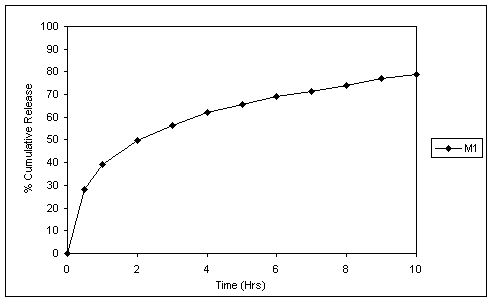
Fig.1 In vitro release profile of matrix tablets without pH adjuster.
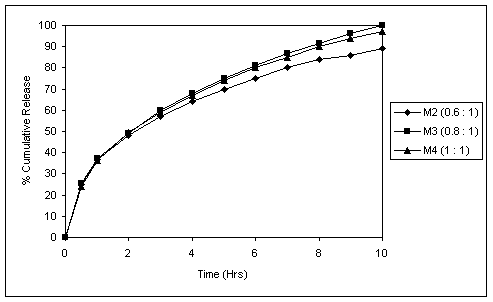
Fig. 2 In vitro release profiles of matrix tablets with different
Succinic acid : drug ratios.
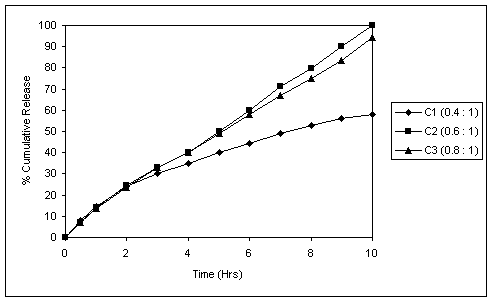
Fig. 3 In vitro release profile of coated tablets (5.8% weight rise,
RSPO : RLPO=9:1) with different Succinic acid : drug ratios.
References:
1. Florey, K.F., (1988) Analytical profiles of drug substances, Vol.-17, 661-662.
2. Ritchel, W. A. (1989), Biopharmaceutic and pharmacokinetic aspects in the design of controlled release peroral drug delivery systems, Drug Dev. Ind. Pharm., 15, 1073-1103.
3. Sanghvi, N. et. al (1994), Controlled drug delivery of pH-dependent soluble drug – Pindolol, Drug Dev. Ind. Pharm, 20, 111-118.
4. Streubel, A. et. al, (2000), pH-independent release of a weakly basic drug from water insoluble and soluble matrix tablets, J. Control Rel., 67, 101-110.
5.
United State Pharmacopoeia 25/National Formulary 20, (2002), US Pharmacopoeial
convention, Inc,
First Published November 2003
All pages copyright ©Priory Lodge Education Ltd 1994-2004.