Clozapine-Associated Elevation of Hepatic Enzymes
Craig B. Franke, M.D.
Resident in Psychiatry
Scott & White - Texas A&M University Health Science Center
CASE PRESENTATION
Mr. H is a 24 year-old Caucasian gentleman in college who was previously diagnosed with Bipolar Disorder, type 1, most recent episode manic with psychotic features, by his outpatient psychiatrist. The patient was initially started on ziprasidone 160 m.g. p.o. nightly. However, he subsequently began to exhibit paranoid delusions, believing that his food was poisoned and that his parents were attempting to take his life. Due to acute agitation, Mr. H was hospitalized. At the time, he reported a history of mood lability, racing thoughts, distractibility and hyper-religiosity. Ziprasidone was discontinued and replaced with lithium carbonate and risperidone during his hospitalization. His condition improved and he was discharged.
However, two weeks later, he complained of sedation and tremor. Lithium was reduced and risperidone was continued. His parents noted that he was noncompliant with his medications and had again become increasingly psychotic, expressing suspiciousness about his family. The patient was disorganized in his thoughts, exhibiting thought blocking with minimal speech. Though he denied hallucinations, he often appeared distracted. He was again hospitalized and lithium and risperidone were continued. However, he developed extrapyramidal symptoms on risperidone, which was then discontinued along with lithium. He was then started on quetiapine. However, he continued to display psychotic symptoms. Ultimately, quetiapine was replaced with clozapine which was titrated up to 200 m.g. nightly with significant improvement in his psychotic symptoms.
Shortly after the initiation of clozapine, routine laboratory studies revealed an acute and significant elevation of the patient’s previously normal hepatic enzymes (figures 1, 2 & 3) with no associated symptoms or other abnormalities noted on laboratory. Prothrombin time (PT) was 12.8 seconds, activated partial thromboplastin time (PTT) was 30.2 seconds and international normalizing ratio (INR) was 1.0. Gastroenterology was consulted and a multitude of studies were ordered including assays for autoimmune hepatitis, as well as a ceruloplasmin for Wilson’s disease, an iron panel to rule out hemochromatosis, a viral hepatitis panel, and alpha-1 antitrypsin. All studies were negative. It was felt that his elevated liver enzymes were likely secondary to clozapine, which was then discontinued. Following discontinuation, the patient’s enzymes returned to the normal range. At follow-up, his hepatic functions remained within normal limits.
DISCUSSION
Clozapine is often considered the prototype of all atypical antipsychotics with demonstration of superiority to traditional neuroleptics in the treatment of refractory schizophrenia and other psychotic symptoms (Kane, Honigfeld, Singer and Meltzer, 1988). Since it first introduction, complications have been associated with its use. Clozapine can cause agranulocytosis, seizures, hypotension, tachycardia, weight gain and sialorrhea. Most adverse-effects may be predicted by its receptor-binding profile. Clozapine has relatively high affinity for cortical Dopamine (D4) compared to D2 receptors as well as significant serotonergic (5-HT2), adrenergic (α1and α 2), muscarinic, and histaminergic (H1) blockade (Van Tol, Bunzow, Guan, Sunahara, Seeman, Niznik and Civelli, 1991), (Baldessarini and Frankenburg, 1991). However, hepatotoxicity associated with the use of Clozapine, though documented, is not a well-known occurrence. The manufacturer’s product information (1996) states a 1% incidence of hepatic function abnormalities and less than 1% incidence of hepatitis and pancreatitis.
The consensus amongst most investigators is that, in the absence of physical signs or symptoms, acute elevations in liver functions with the use of clozapine are likely a biochemical abnormality rather than an indication of necrotic liver injury. Elevations are often mild, benign, and transient (Young, Bowers and Mazure, 1998). One case reported clozapine-associated elevation of liver function tests (LFT). In this instance, elevated enzymes were noted at approximately 5 weeks with a dose of 300-500 m.g. daily. Following discontinuation, LFTs returned to normal values at 5 weeks. The authors recommended routine LFTs at 5 weeks after starting clozapine (Markowits, Brinberg and Jackson, 1997).
In a rare and unusual case, MacFarline, Davies, Mannan, Sarsam, Pariente and Dooley (1997) reported fatal acute fulminant liver failure in a 30-year-old Asian man on clozapine. Liver biopsy indicated zonal liver necrosis consistent with drug or toxin injury. After eight weeks at 350 m.g. per day maintenance, the patient experienced symptoms of nausea, vomiting, abdominal pain, jaundice and dizziness. No other cause could be determined on history, clinical examination, or laboratory data. Multi-system organ failure during clozapine use has also been described in a case report of a 23-year-old female. Dosing was started at 12.5 m.g. daily and increased to 100 m.g. daily over five days. There was a 26-day delay prior to onset of symptoms. The authors speculated that this delay might reflect activation of the cytochrome P450 system of the drug to its reactive metabolites 8.
The relationship of dose and hepatoxicity has been examined. In a four year follow-up study, Marinkovic, Timotijevic, Babinski, Totic and Paunovic (1994) suggested that the increase of liver enzymes associated with clozapine is dose-related since the reduction of daily dose resulted in normalization of enzymes 9. Conversely, in a prospective study, inpatients on clozapine were compared to a control group of inpatients on haloperidol. Only patients with normal liver enzymes prior to participation and without a history of alcohol use were included. Liver functions were monitored weekly for 18 weeks. The most common abnormality was an isolated asymptomatic increase in the aminotransferase levels with alanine aminotransferase (ALT) increased most frequently. Clozapine patients showed a greater frequency of increased ALT (37.3%) relative to those treated with haloperidol (16.6%). The investigators found that clozapine plasma levels and male gender had a direct and positive association with elevated liver enzyme but no dose-dependency was observed. Furthermore, liver enzymes spontaneously normalized (60%) without jaundice after 13 weeks of continuous treatment 10. Patton, Remick, and Isomura (2000) also described clozapine-associated elevation of liver enzymes as dose-independent (Patton, Remick and Isomura, 2000).
Some patients may benefit from re-challenging of clozaril following transient elevations of liver enzymes with initial exposure to the drug. Clozapine was titrated to 500 m.g. daily over 8 weeks in a 40-year-old male inpatient with concomitant use of lithium carbonate. After 2 weeks, LFTs were elevated with marked increases in transaminases, but no symptoms were evident. Liver enzymes returned to normal 30 days following the discontinuation of clozapine. The patient was re-challenged with a gradual increase in the dose over one month to 300 m.g. daily. Mild, but transient increases in aspartate aminotransferase (AST) and ALT were noted with no clinical signs or symptoms. Over the next 6 weeks, the dose was increased to 600 m.g. daily, in divided doses. At one-year follow-up, LFTs remained with in normal limits (Eggert, Crismon, Dorson and Taylor, 1994).
Similarly, a retrospective study reviewed 7,263 treatment courses of four neuroleptics as monotherapy. Of the cases examined, 1,280 were treated with clozapine, 2,667 with haloperidol, 917 with perphenazine, and others with perazine (a frequently used neuroleptic in Germany). Investigators controlled for previously elevated LFTs, co-administration of other medications that could elevate liver enzymes, and treatment of less than 3 weeks. Over a course of 12 months, liver enzymes were serially obtained. Clozapine was associated with the greatest incidence of any elevation of liver enzymes (78%). Spontaneous normalization of liver enzymes occurred in more than 50% of clozapine patients without the daily dose being changed. Dose reduction had no effect on the time required for normalization (Gaertner, Altendorf, Batra and Gaertner, 2001).
In summary, clinical evidence suggests that elevations in liver transaminases up to three times the normal limits should not prevent further treatment with clozapine. Furthermore, an asymptomatic 3-fold or greater increase in ALT is most often associated with clozapine dosed at 300 m.g. to 500 m.g. Other possible causes of elevated LFTs should be excluded, given that hepatotoxicity is often unpredictable, occurring in the first week of treatment, but may not appear until 8 weeks (Erdogan, Kocabasoglu, Yalug, Ozbay and Senturk, 2004). Patients may benefit from continued treatment without dose reduction since reports suggest a dose-independent relationship of clozapine to increases in liver enzymes. Reduction in dose does not appear to reduce the time required for normalization of LFTs with spontaneous remission reported at 13 weeks with or without continuation of medication. Routine monitoring of LFTs at 5 weeks after starting clozapine with regular follow-up laboratory in symptomatic patients may be of benefit in early identification of those at risk for further, more serious hepatic complications. Despite current knowledge, further investigation is required to establish clearer guidelines regarding management of clozapine-associated elevation of liver enzymes.
References
Baldessarini, RJ and Frankenburg, FR (1991). Clozapine: a novel antipsychotic agent. New England Journal of Medicine. 324, 746-754.
Eggert, AE, Crismon, ML, Dorson, PG and Taylor, RL (1994). Clozapine rechallenge after marked liver enzyme elevation. Journal of Clinical Psychopharmacology. 14(6), 425-426.
Erdogan, A, Kocabasoglu, N, Yalug, I, Ozbay, G and Senturk, H (2004). Management of marked liver enzyme increase during clozapine treatment: a case report and review of the literature. International Journal of Psychiatry in Medicine. 34(1), 83-89.
Gaertner, I, Altendorf, K, Batra, A and Gaertner, HJ (2001). Relevance of liver enzyme elevations with four different neuroleptics: a retrospective review of 7,263 treatment courses. Journal of Clinical Psychopharmacology. 21(2), 215-222.
Hummer, M, Kurz, M, Kurzhaler, I., Miller, C, Oberbauer, H and Fleischhacker, WW (1997). Hapatotoxicity of clozapine. Journal of Clinical Psychopharmacology. 17(4), 314-7.
Kane, J, Honigfeld, G, Singer, J and Meltzer, H (1988). Clozaril collaborative group: clozapine for treatment-resistant schizophrenia: a double-blind comparison with chlorpromazine. Archives of General Psychiatry. 45, 789-796.
MacFarline, B, Davies, S, Mannan, K, Sarsam, R, Pariente, D and Dooley, J (1997). Fatal acute fulminant liver failure due to Clozapine: a case report and review of Clozapin-induced hepatotoxicity. Gastoenterology. 112, 1707-1709.
Marinkovic, D, Timotijevic, I., Babinski, T, Totic, S, and Paunovic, V (1994). The side-effects of clozapine: a four year follow-up study. Progress in Neuro-psychopharmacology and Biological Psychiatry. 18(3), 537-544.
Markowits, JS, Brinberg, R and Jackson, C (1997). Marked liver enzyme elevations with clozapine. Journal of Clinical Psychopharmacology. 17(1), 70-71.
Patton, S, Remick, RA, and Isomura,T (2000). Clozapine; an atypical reaction. Canadian Journal of Psychiatry. 45(4), 393-394.
Product information (1996). Sandoz Pharmaceuticals Corporation, East Hanover, NJ.
Van Tol, HHM, Bunzow, JR, Guan, HC, Sunahara, RK, Seeman, P, Niznik, HB and Civelli, O (1991). Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 350, 610-614.
Young, CR, Bowers, Jr., MB and Mazure, Carolyn M (1998). Management of the adverse effects of clozapine. Schizophrenia Bulletin. 24(3), 381-390.
Figure 1: Graph of AST.
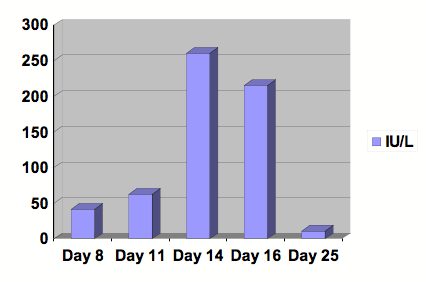
Serial studies were drawn beginning at day 8 following the initiation of Clozapine. After 13 days of treatment, Clozapine was discontinued. Day number 25 was at outpatient follow-up. Reference range: 0-40 IU/L.
Figure 2: Graph of ALT.
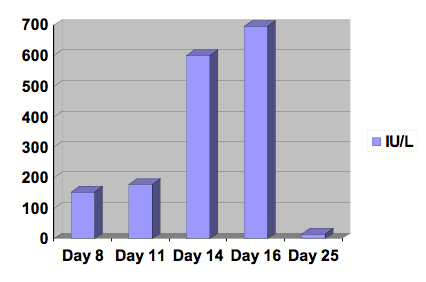
Serial studies were drawn beginning at day 8 following the initiation of Clozapine. After 13 days of treatment, Clozapine was discontinued. Day number 25 was at outpatient follow-up. Reference range: 0-29 IU/L.
Figure 3: Graph of Alkaline Phosphatase.
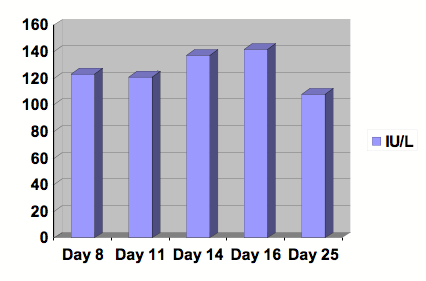
Serial studies were drawn beginning at day 8 following the initiation of Clozapine. After 13 days of treatment, Clozapine was discontinued. Day number 25 was at outpatient follow-up. Reference range: 30-115 IU/L.
First Published February 2007
All pages copyright ©Priory Lodge Education Ltd 1994-


