Browse through our Medical Journals...
Temporal-illusory conjunction in schizophrenia.
Hiroshi Watanabea, Atsushi Yoshikawab, Masayuki Kinutanib, Fujihiko Ohtsukib, Kazuyuki Oharab, Norio Uenob, Kazutoshi Takedab, Hana Nishiokab, Hirokazu Yamamotob, Hiroyuki Umemuraa, and Katsunori Matsuokaa
a The National Institute of Advanced Industrial Science and Technology, Institute for Human Science and Biomedical Engineering, Osaka 563-8577, Japan
b Arima-Kogen Hospital, Hyogo, 651-1512, Japan
Corresponding Author:Hiroshi Watanabe The National Institute of Advanced Industrial Science and TechnologyInstitute for Human Science and Biomedical Engineering
1-8-31 Midorigaoka, Ikeda, Osaka 563, Japan
Phone: +81-727-51-9534;
email: h.watanabe@aist.go.jp
Abstract
In this paper, we wish to clarify the visual perceptions of schizophrenic patients, which were obtained using rapid, serial visual inputs. The patients observed a stream of colored letters in the same position on a display and were first asked to name the red letter and then to find the 'X' in the same stream of letters, as quickly as possible (dual task). Our results showed that they more frequently made illusory conjunctions of shape and color rather than the healthy control subjects. However, the schizophrenic group performed as well as the control group when they were not required to find the 'X'.
We discussed the patients' attention-control and temporal-management dysfunctions for the lower level visual input.
INTRODUCTION
Recently, the cognitive abilities of schizophrenic people have been extensively studied using the psychophysical method. In addition to the higher-order cognitive dysfunctions, (contextual memory, Water et al., 2004; recognition of facial emotional expressions, Suslow et al, 2003; and categorical judgments, Sumiyoshi et al., 2002), some patients have demonstrated dysfunctional processing of low-level information, such as color and luminance (Schechter et al., 2003; McClure, 2001), working memory (Jansma et al., 2004; Goldman Rakic, 1994), and attention (Cheung et al., 2000; Li et al., 2002; Myles-Worsley et al., 1991). In the area of attention, the temporal abilities have not been investigated as thoroughly as the spatial properties of schizophrenics. Cheung et al. (2000) and Li et al. (2002) reported the amount of attentional resources and the temporal abilities required to recover the exhausted attentional resources, using the rapid serial visual presentation (RSVP) paradigm with the signal detection theory. We focused on the process of integrating visual properties, such as shape and color, by presenting colored letters in the same positions, serially and rapidly (RSVP paradigm). Our main purpose was to clarify the schizophrenics' abilities to visually representation obtained from serial and rapid visual input, based on the quality and quantity of the errors, and not the accuracy of their performance. We found that the schizophrenic group made more systematic errors than the healthy subjects, which seems to suggest a distorted visual representation.METHODS
Subjects:The subjects were made up of twenty Japanese healthy and twenty Japanese schizophrenic subjects. The healthy control subjects were paid a fee for their participation and were recruited from Kobe University, Kobe, Japan. All schizophrenic subjects fulfilled the diagnosis of schizophrenia defined by the Brief Psychiatric Rating Scale (BPRS) and their averaged score and SD were 42.93 and 3.30, respectively. The diagnosis of schizophrenia was made based on clinical interview and review of medical records by senior psychiatrists with consensus. All subjects were aged between 20 and 58 years with normal or corrected to normal visual acuity and normal color vision. All experimental procedures were approved by the Ethics Committee for Human and Animal Research of Human Stress Signal Research Center in National Institute of Advanced Science and Technology.
Apparatus: The stimuli were generated and answers were recorded using an IBM-compatible DOS/V computer with a 17-inch color monitor. The monitor had a 12-ms response time and the vertical refresh rate was 75 Hz. The screen display resolution was 1,280 x 1,024 dots (SXGA). The subjects used a mouse and the tester used a keyboard to respond to and record the task.Experimental design: The experiment consisted of the following two tasks; a single task naming a colored letter; and a dual task, detecting a shape and naming the color. In each task, eighteen (or seventeen according to the conditions described below) uppercase letters (A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P, Q and X) appeared in the center of the screen in pseudo-random order. We also set two stimulus onset asynchrony (SOA) conditions of 200 and 150 ms. The inter-stimulus interval (ISI) was 50 ms for each SOA condition (Figure 1).
Figure 1
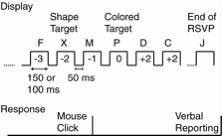
In the single task, the 7th, 8th , or 9th letter of the RSVP stream was red. The colors of the other letters were randomly selected from five colors (green, blue, white, yellow, and cyan). The subject's task was to name the red letter verbally after observing the entire RSVP stimulus set. The tester typed their responses using the keyboard. Occasionally, 'X' was included the RSVP stream, at one of eight possible positions from the red letter in the RSVP stream. These nine possibilities were repeated three times, and the subjects observed twenty-seven trials for each SOA condition. The order of the 'X' position (or no 'X') was random. However, we did not inform the subjects in advance about the 'X' and simply asked them to name the red letters. Therefore we believe that the position of 'X' did not affect the performance. We treated this task as the control task in the dual tasking situation. In the dual task, the subjects were asked to click the right mouse button as quickly as possible when they found the 'X' in the RSVP stream, in addition to the single task. The subjects conducted twenty-seven trials for each SOA condition, similar to the single task.
Procedure: The subjects were seated in a dark room, approximately 43-cm away from the monitors. Their heads were not restrained, but we inserted a calibration mark on the display to adjust their eye position to the center of the display, horizontally and vertically, at the beginning of each task. Under these viewing conditions, the display and stimuli were approximately arranged at visual angles of 50.0 x 38.5, 4.7 x 4.5 degrees, respectively. The tester explained the task before the start of each task, and asked subjects to practice a few trial runs under SOA = 350-ms conditions. The subjects participated in the two tasks under the two SOA conditions and did 108 trials ((27+27)*2). Two tasks were given in a fixed order (from single task to dual task), and the order of the two SOA conditions was randomly selected in each task.
RESULTS
Our main purpose was to clarify the temporal illusory conjunction of the visual abilities of the schizophrenic group. Previous studies (Cheung et al., 2000; Li et al., 2002) dealt with a phenomenon called 'attentional blink', and discussed the schizophrenics' ratio of correct responses for the second target, which decreased as the time lag between the prior and posterior target shortened. Our results were similar to their findings (i.e. the amout of attentional blink for the second target were higher in schizophrenics than in healthy subjects, but the schizophrenic subjects needed a longer interval between the letters to recover the baseline). Here, we omitted the details and focused on the error properties.
Tables 1 show that the relative frequency in which the subjects saw a red letter at each relative distance from the correct position of the red letter, in both the single and dual tasks under SOA=150msec condition. Under SOA=200msec condition, it was difficult to find the large effect of experimental design (schizophrenia vs. healthy, dual task vs. single task.) on the relative frequency and we did not show the table. The healthy subjects performed almost completely under that SOA condition in both single and dual task. The schizophrenic subjects showed a little meaningful changes between single and dual task, and we referred this point in the following text.Table 1
The relative frequency of illusory conjunction in SOA=150msec.
Dual Task
Reported position of red letter to the simulated position |
||||||||||||
| Control | -5 | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | |
Lag (X-Red) |
-600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| -450 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| -300 | 0.00 | 0.06 | 0.00 | 0.00 | 0.03 | 1.00 | 0.11 | 0.00 | 0.03 | 0.00 | 0.00 | |
| -150 | 0.10 | 0.00 | 0.03 | 0.00 | 0.00 | 1.00 | 0.17 | 0.14 | 0.03 | 0.00 | 0.00 | |
| 150 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 450 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.03 | |
| 600 | 0.03 | 0.00 | 0.03 | 0.03 | 0.00 | 1.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | |
| Schizo. | -5 | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | |
Lag (X-Red) |
-600 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 1.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| -450 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 1.00 | 0.08 | 0.04 | 0.00 | 0.04 | 0.00 | |
| -300 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 1.00 | 0.15 | 0.23 | 0.00 | 0.00 | 0.00 | |
| -150 | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 1.00 | 0.71 | 0.00 | 0.43 | 0.00 | 0.00 | |
| 150 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | |
| 300 | 0.00 | 0.00 | 0.00 | 0.08 | 0.08 | 1.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | |
| 450 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 1.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
Single Task
Reported position of red letter to the simulated position |
||||||||||||
| Control | -5 | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | |
Lag (X-Red) |
-600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| -450 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| -300 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 1.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |
| -150 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 150 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 450 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 1.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | |
| 600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Schizo. | -5 | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | |
Lag (X-Red) |
-600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| -450 | 0.04 | 0.00 | 0.00 | 0.04 | 0.04 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | |
| -300 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| -150 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 150 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 300 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 1.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | |
| 450 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 600 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 1.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | |
Each row represents the time lag between the X and red letters; the negative values mean that X appeared before the red letter (from –600- to –150-ms in Table 1), the positive values mean that the X appeared after the red letter (from 150- to 600-ms in Table 1). Each column represents the plus-minus five positions around the correct position (0) of the red letter, and responses outside this range were omitted. Each value in the cells shows the relative error frequency in which the numbers reported for each position were divided by the number reported for the correct position where the red letter appeared. Therefore, column '0' was only filled with 1.0 when the subjects completed the task. The values were reversed and increased in the other cell, because the frequency of illusory conjunction was observed more often. For example, the healthy subjects had a almost 100% accuracy rate for reporting the red letter in the single task, regardless of the SOA conditions.
The schizophrenic subjects, on the other hand, had the highest ratio of illusory conjunctions when the X appeared immediately before the red letter (lag = -150 ms). In this case, the relative ratio for reporting the letter as red after +1 and +3 of the correct letter rose to as high as 71% and 43%, respectively. Under the SOA=200msec condition, they showed the similar tendency when the X appeared immediately before the red letter (lag = -200 msec). In this case, the relative ratio for reporting the letter as red after +1, +2 and +3 of the correct letter rose to as high as 11%, 11% and 17%, respectively. Our results, qualitatively suggest that the ratio of illusory conjunctions was higher in the schizophrenic group than the healthy group for the dual task rather than the single task, and for the shorter SOA rather than the long SOA. This also increased when X appeared before the red letter. However, almost all the illusory conjunction ratios were less than 10%, and the performance of schizophrenic group seemed to deteriorate when we overloaded the tasks (the dual task and short SOA).
DISCUSSION
The some illusory conjunction models in healthy people assumed the phenomenon, based on the different processing speeds of the visual modules (filter model, Broadbent, 1977 ; and parallel model, Keele et al., 1978). This difference caused the failure of the synchronous output for each visual module (color, shape, etc.) and bound the visual properties. For example, when the color 'red' (the property of the target definition) was detected, the next letter was buffered and was reported as a red letter. The SOA used in this experiment was much longer than in previous studies that used healthy subjects (Chun, 1977), allowing these subjects to process and bind the visual properties well, even when performing the dual task.
Neuroscientific findings prior to cortical processing, suggest that the retinal output comes from magno and parvo systems, which have different responses. The magno system responds transiently to the appearance of the stimulus, regardless of the wavelength. The parvo system responds slowly and sustains the response to examine the properties of the stimulus. Some neuropsychological studies show a dysfunction in the initial stages of visual information processing in schizophrenics. In these studies, two stimuli were presented serially at the same position using a visual backward masking (VBM) task, in which the first stimulus is covered by the second stimulus. In schizophrenics, visual targets require a longer time to 'erase' the effects of subsequent masks. In general, schizophrenic patients require approximately 300 ms between the target and mask to successfully identify the target; whereas healthy subjects need only about 120 ms.
These VBM deficits in schizophrenics have been attributed to the hyper-activity of the transient channel, leading to greater transient channel interference with ongoing sustained channel processing, Schechter et al., 2003; Weiner et al., 1990). Our results, however, cannot be explained using only these magno-parvo system viewpoints, because the data from our schizophrenic group showed that the adjacent letters did not interfere with the detection of the target stimulus (a red letter to be named) under single-task conditions. In other words, the VBM did not occur because the SOAs used in this experiment were too short (200- and 150-ms) to cause the VBM effect (Schechter et al., 2003; Braff et al.; 1991; Weiner et al., 1990).
This discrepancy might due to differences in the luminance of the stimuli, which in this experiment were too high to activate the magno system, causing the parvo system to do most of the processing, producing results that were equivalent to the results observed in the healthy subjects (Schechter et al., 2003). Therefore, the results from our singular task in the schizophrenic group might reflect on the disordered integration of the serial target definition.
Instructions were only issued for the target stimuli (red letter) for the single task, so the subject could ignore everything except the target stimuli. The results suggest that both groups showed the same ability to ignore the non-essential letters. However, the schizophrenic group showed the illusory conjunction when the X appeared before the red letter. Some of previous studies suggested that attention played an important roll in feature integration (Treisman, 1998; Treisman et al., 1980). The temporal order of each visual property must be considered to obtain an integrated visual representation after the individual visual modules successively process the multiple properties.
Our results suggested that the schizophrenic subjects seemed dysfunctional in the 'time management' of the order of the visual properties, or in release their attention for the first stimulus after identifying it. Particularly in the time management aspect of the tasks, the schizophrenic person might transfer the distorted representation to the environment. However, more examination is needed to deal with the temporal aspects of activation and/or inhibition in the central nervous system.
CONCLUSION
In conclusion, we compared the temporally successive visual representations obtained from schizophrenic and the healthy subjects. The results suggest that both groups performed the simple naming task the same, and their visual information processing was not significantly different at the single task level. In the dual task, on the other hand, we found that the schizophrenic group exhibited illusory conjunction of visual properties in addition to the attentional blinks previously reported. These results suggest depression of the attentional functions and a dysfunction in temporally processing visual properties in the schizophrenic group.
REFERENCES
Braff, DL, Saccuzzo, DP, Geyer, MA, (1991) Information processing dysfunctions in schizophrenia: studies of visual backward masking, sensorimotor gating, and habituation. In: Steinhauer, SR, Gruzelier, JH, Zubin, J. (Eds.), Handbook of Schizophrenia, 5. Elsevier, New York , 303-334.
Broadbent, DE, (1977) Colour, localisation and perceptual selection. Psychologie Exmerimentale, et Comparee, Presse Universitaire de France, Paris, 95-08.
Cheung, V., Chen, EYH, Chen, RYL, Woo, MF, Yee, BK, (2000) Evidence for normal temporal inhibition of visual attention in schizophrenia: a comparative study in inpatients and healthy subjects on attentional blink. Schizophrenia Research 41, 296-297.
Chun, MM, (1997) Temporal binding errors are redistributed by the attentional blink. Perception and Psychophysics 59, 1191-1199.
Goldman Rakic, PS, (1994) Working memory dysfunction in schizophrenia. Special issue: the frontal lobes and neuropsychiatric illness. Journal of Neuropsychiatry and Clinical Neurosciences 6, 348-357.
Jansma, JM, Ramsey, NF, van der Wee, NJA, Kahn, RS, (2004) Working memory capacity in schizophrenia: a parametric fMRI study. Schizophrenia Research 68, 159-171.
Keele, SW, Neil, WT, (1978) Mechanisms of attention. In: Carterette, EC, Friedman, MP (Eds.): Handbook of Perception IX. Academic Press, New York.
Li, CR, Lin, W., Yang, Y., Huang, C., Chen, T., Chen, Y., (2002) Impairment of temporal attention in patients with schizophrenia. Neuroreport 13, 1427-1430.
McClure, RK, (2001) The visual backward masking deficit in schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 25, 301-311.
Myles-Worsley, M., Johnston, WA, Wender, PH, (1991) Spontaneous selective attention in schizophrenia. Psychiatry Research 39, 167-179.Schechter, I., Butler, PD, Silipo, G., Zemon, V., Javitt, DC, (2003) Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophrenia Research 64, 91-101.
Sumiyoshi, C., Matsui, C., Sumiyoshi, T., Yamashita, I., Sumiyoshi, S., Kurachi, M., (2002) Semantic structure in schizophrenia as assessed by the category fluency test: effect of verbal intelligence and age of onset. Psychiatry Research 105, 187-199. Suslow, T., Roestel, C., Ohrmann, P., Arolt, T., (2003) Detection of facial expressions of emotions in schizophrenia. Schizophrenia Research 64, 137-145.
Treisman, AM, (1998) Feature binding, attention and object perception. Philosophical Transactions of the Royal Society of London B, 353, 1295-1306,
Treisman, AM, and Gelade, G., (1980) A feature-integration theory of attention. Cognitive Psychology 12, 97-136.
Waters, FAV, Maybery, MT, Badcock, JC, Michie, PT, (2004) Context memory and binding in schizophrenia. Schizophrenia Research 68, 119-125.Weiner, RU, Opler, LA, Kay, SR, Merriam, AE, Papouchis, N., (1990) Visual information processing in positive, mixed, and negative schizophrenic syndromes. The Journal of Nervous and Mental Disease 178, 616-626.
First Published April 2005
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


