Browse through our Medical Journals...
CLINICAL SIGNS DUE TO BLOOD PARASITES IN FALCONS
Walter Tarello, DVM, MRCVS
Corresponding author: C.P. 1644, 06129 Perugia ITALY
Abstract
Specific and non-specific clinical signs are documented in infections due to blood parasites such as Haemoproteus tinnunculi, Leucocytozoon toddi, Babesia shortti, Aegyptianella spp. and Ehrlichia spp. in captive birds of prey. However, written reports very rarely hold the strength of depiction inherent to photographs and movies. Common and unusual signs occurring in falcons carrying intracellular haematozoa are reported here with the help of movies and photos.
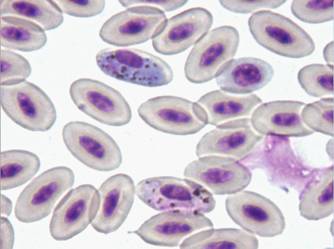
1 - Haemoproteus tinnunculi gametocytes in a captive falcon
Babesiosis
TABLE 1 - CLINICAL SIGNS ASSOCIATED WITH BABESIA SHORTTI INFECTION IN 21 FALCONS (Tarello, 2006a)
N %
CLOSED EYES (Somnolence) 14 66.6
WEIGHT LOSS 12 57.1
ANOREXIA/POOR APPETITE 7 33.3
LETHARGY 7 33.3
VOMITING 6 28.6
GREEN FECES (Blood in stool) 6 28.6
POOR COORDINATION 6 28.6
SEIZURE 6 28.6
REDUCED SPEED & STRENGTH IN FLIGHT 6 28.6
LOWERED HEAD 5 23.8
CONTINUOUS CRYING 5 23.8
DYSPNOEA 4 19.0
DIARRHOEA 4 19.0
COLLAPSE & STATE OF SHOCK 1 4.7
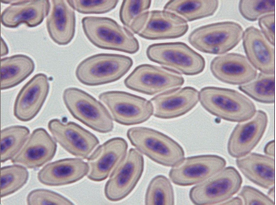
Diagnosis of Babesia shortti in Falconiformae is made by demonstrating the round to oval, 0.7-2.4 µm in size, non-pigmented parasites in the cytoplasm of red blood cells stained with Giemsa, Wright or Diff-Quick techniques. They usually cause displacement and/or rotation of the host cell nucleus.
The intensity of parasitaemia varies greatly. The organism is not always apparent in the bloodstream and it might be necessary to examine several smears to establish its presence (Remple, 2004). Number of reports on babesiosis in raptors has recently increased (Samour, 2005). However, falcon babesiosis is probably more common than reported because of the rarity of the organism in peripheral blood smears and because avian Babesiae are often confused with early stages of other haematozoa such as Plasmodium and Haemoproteus or artefacts (Peirce, 2003; Tarello, 2006a).
Clinical manifestation of babesiosis varies from sub-clinical signs in apparently healthy animals with low parasitaemia to severe disease associated with high parasitaemia. Diagnosis of avian babesiosis due to Babesia shortti was recently done in 21 (1.54%) of 1360 diseased falcons (Tarello, 2006a). Clinical signs were present even in cases showing very low Babesia density: closed eyes, weight loss, anorexia, lethargy, vomiting, seizure, lowered head and blood in the stool (Table 1).

The clinical sign ‘continuous crying’, observed in 23.8% of patients (Table 1), is probably due to severe muscular and joint pain associated with high babesial parasitaemia and manifest itself as seen in Movie 1).
Following treatment with imidocarb dipropionate (Imizol®, Schering-Plough Animal Health Corp, Union, NJ, USA) at the same dosage recommended for dogs (i.e. 7.13 mg/kg, given intramuscularly once a week for 3 weeks), rapid resolution of clinical signs is usually observed. Imidocarb dipropionate seems very effective in the treatment of Babesiae in raptors (Movie 2).
Patent infection in adult birds without previous exposure to babesiae can produce state of shock (4.7%), seizures (28.6%) and ‘closed eyes (somnolence)’ (66.6%)(Movie 3).
Aegyptianellosis
Table 2 – SIGNS ASSOCIATED WITH AEGYPTIANELLA INFECTION IN 35 FALCONS (Tarello, 2006b)
No. %
WING/LEG ARTHRITIS 17 48.5
REDUCED SPEED & STRENGTH IN FLIGHT 16 45.7
WEIGHT LOSS 16 45.7
DYSPNOEA 16 45.7
DIARRHOEA 11 31.4
ANOREXIA/POOR APPETITE 11 31.4
WEAKNESS 7 20.0
SINUSITIS 5 14.3
LETHARGY 4 11.4
VOMITING 4 11.4
BUMBLEFOOT 4 11.4
BLOOD IN THE STOOL 3 8.6
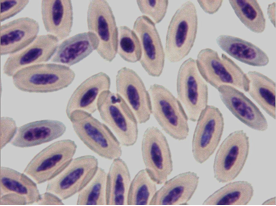
Bacteria of the genus Aegyptianella sp. (Anaplasmataceae) are tick-transmitted rickettial parasites of avian erythrocytes causing a disease in birds similar to anaplasmosis in cows. Aegyptianella organisms range from 0.5 to 3 μm in size (Fig. 5-6).
![]()
Microscopic detection of round (Fig. 5), oval and clover-like (Fig. 6-8) inclusion bodies morphologically consistent with Aegyptianella and located internally to, protruding from (Fig. 7) or entering/exiting the erythrocytes (Fig. 5) is diagnostic for aegyptianellosis. These rickettsial forms can be seen either inside or outside the red blood cells membranes, since erythrocytic vesiculation is the mode of entrance or exit of initial bodies from the red cells (Tarello and Riccieri, 2003).
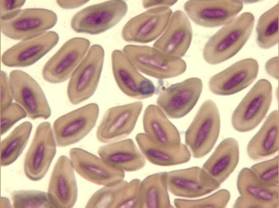
Fig. 7 – Large Aegyptianella inclusion body protruding from a red cell wall
Common clinical signs are arthritis, reduced speed and strength in flight, dyspnoea and diarrhea (Table 2)(Tarello, 2006b). In Italy, a heron and two birds of prey diagnosed with aegyptianellosis showed anorexia, emaciation, paralysis, diarrhoea, fever and death (Tarello, 2001; Tarello and Riccieri, 2003). Young animals, immune-depressed adults or birds living in non-endemic areas can develop acute infection leading to sudden death. Severe ataxia and weakness (20 %) is reported during heavy infections (see Movie n. 4).
-
Response to doxycycline treatment at rickettial doses (25 mg/kg/day for 21 days) is consistent with the cytological observation of aegyptianellosis and compatible with the suggested criteria for its diagnosis. Movie n. 5 (below) shows the same falcon after one week of doxycycline therapy.
-
The geographical distribution of aegyptianellosis follows the distribution of its vector tick, Argas persicus, mostly in tropical and subtropical areas, including some countries of southern-eastern Europe (Peirce and Bevan, 1977).
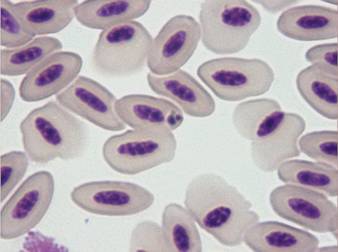
Fig. 8 – Clover-like inclusion body typical for Aegyptianella
Haemoproteus
Table 3
CLINICAL SIGNS ASSOCIATED WITH HAEMOPROTEUS TINNUNCULI INFECTION IN FALCONS
(Tarello, 2007)
REDUCED SPEED & STRENGTH IN FLIGHT 28 43.1
WEIGHT LOSS 19 29.2
ANOREXIA/POOR APPETITE 17 26.1
DYSPNOEA 14 21.5
LETHARGY 14 21.5
WING ARTHRITIS 10 15.4
REPORTEDLY NON SYMPTOMATIC 8 12.3
SWOLLEN EYES/FEET 5 7.7
ATAXIA 5 7.7
VOMITING 4 6.2
CLOSED EYES 4 6.2
STOP/DELAYED MOULT 4 6.2
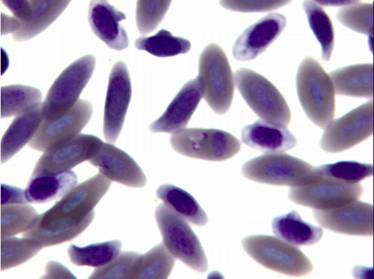
The avian malaria parasite Haemoproteus tinnunculi (Fig. 9-10) is transmitted by the ceratopogonid biting flies of the genus Culicoides (Peirce, 2003). Gametocytes can be detected in blood smears wrapped around the nucleus of infected red blood cells (Lierz et al., 2002) and sometimes the nucleus is displaced by the parasite (Fig. 9).
There are 128 species of Haemoproteus all of which are host-specific to the family level (Bennett et al., 1994). They can affect a variety of birds of prey with multiple infections being of common observation (Lierz et al., 2002).
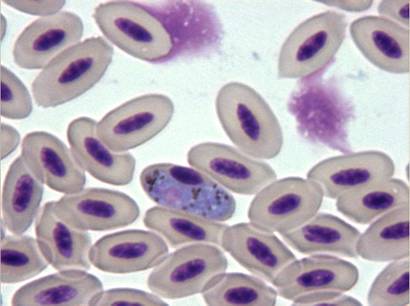
Fig. 9 – Gametocyte of Haemoproteus tinnunculi displacing the nucleus of the red blood cell
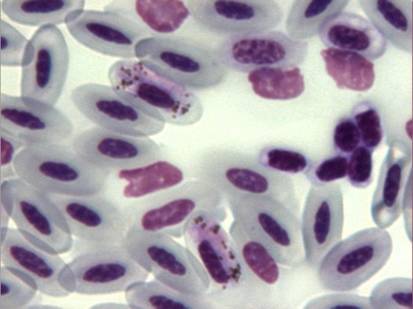
Fig. 10 – Two gametocytes of Haemoproteus tinnunculi not altering the position of the nucleus
Prolonged rehabilitation time and higher mortality rate have been seen in captive raptors with Haemoproteus infection (Olsen and Gaunt, 1985; Ziman et al., 2004).
In the recent past, pathological changes and pathogenic effects have been increasingly reported in association with Haemoproteus infection in a variety of birds (Garvin et al., 2003; Peirce, 2003). Appearance of clinical manifestations depends upon the intensity of infection. The presence of H. tinnunculi was found associated with clinical signs in 87.7% of falcons in the Middle East (Tarello, 2007) (Table 3). Reduced speed and strength in flight (poor performances) was the most common finding (43.1%).
Limb paralysis is a rare unusual sign occurring in falcons heavily infected by Haemoproteus tinnunculi, most probably due to the parasitic vasculitis affecting the small capillary vessels (Reggiardo, 1997) (see Movie 6)-
Following oral treatment with the specific anti-malaria drug named primaquine (0.75 mg/kg for 5 days), falcons experience complete or partial recovery in association with disappearance (20%) or reduction in intensity of parasitaemia (63%), thus confirming that the clinical signs aree linked to the H. tinnunculi infection (Tarello, 2007). Movie n. 7, below, shows the falcon with right limb paralysis 1 week later, after completion of therapy with primaquine:
- Insert MOVIE 7 -
Compared to wild birds, in semi-domesticated animals such as captive falcons, pathogenic effects are easier to detect and are associated with host stress (Heidenreich, 1997). Low intensity of H. tinnunculi parasitaemia is noted in healthy falcons indicating that appearance of symptoms is parasite burden-dependant (Tarello, 2007).
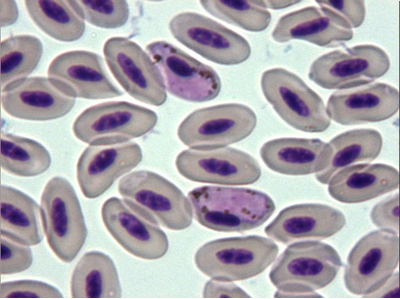
Fig. 11 – Haemoproteus tinnunculi
LEUCOCYTOZOON
Table 4 – SIGNS ASSOCIATED WITH LEUCOCYTOZOON TODDI INFECTION IN FALCONS (Tarello, 2006d)
N %
REDUCED SPEED & STRENGTH IN FLIGHT 9 47.3
ANOREXIA/POOR APPETITE 8 42.1
WEIGHT LOSS 7 36.8
WEAKNESS 6 31.5
DYSPNOEA 5 26.3
CLOSED EYES (Somnolence) 2 10.5
VOMITING 2 10.5
INCOORDINATION/ATAXIA/SEIZURE 2 10.5
BAD FEATHER’S CONDITION/ STOP MOULT 2 10.5
BUMBLEFOOT 2 10.5
DEATH 2 10.5
Pathogenicity associated with haematozoa of the genera Leucocytozoon (Apicomplexa, Haemosporida) has been recorded in domestic waterfowl, turkeys, chickens and pigeons. Signs reported more often are lethargy, emaciation, anorexia, weight loss, anaemia, listlessness, dyspnoea and death (Peirce, 2003).

Fig. 12 –Macrogametocyte of Leucocytozoon toddi in a falcon (x100)
The genera Leucocytozoon includes around 60 species of intracellular parasites belonging to the ‘avian malaria’ complex, transmitted by intermediate hosts such as the ornithophylic black flies Simuliidae (Peirce, 2003). Leucocytozoon toddi has been recently associated with clinical disease and 10% mortality rate in captive birds of prey from Kuwait (Table 4)(Tarello, 2006d). Falcons from Dubai diagnosed with sole leucocytozoonosis exhibited compatible clinical signs such as reduced speed and strength in flight, weight loss, poor appetite, airsacculitis and arthritis. Anaemia is usually present in severe infections and manifests itself with ‘dyspnoea’ (see Movie 8, below):
Bad feather’s condition and stopped moult has been reported in 10% of falcons affected by leucocytozoonosis (Fig.13). This is due to the peculiar attraction for the feather’s pulp localization exhibited by these parasites.

Fig. 13 – Bad feather condition and stopped moult in a falcon with severe leucocytozoonosis
Concomitant disappearance of clinical signs and of haematozoa from the blood was recently obtained with the use of melarsomine (Cymelarsan®, Merial, IM, 0.25 mg/kg/day for 4 days)(Tarello, 2006d). The rational for the use of the organic arsenical melarsomine was based on the assumptions that current anti-malaria drugs are not effective in eliminating Leucocytozoon spp. (Remple, 2004) and that a variety of arsenic-based medicaments has been successfully used against malaria during the last two centuries (Tarello, 2006d).

Fig. 14 – Feathers are growing back, 45 days after melarsomine therapy, in the same falcon of picture 13.
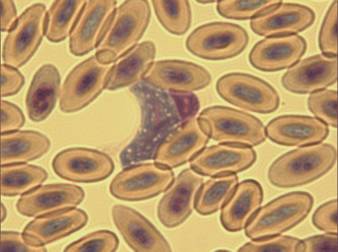
Fig. 15 – Leucocytozoon toddi
EHRLICHIA
Diagnosis of ehrlichiosis in birds is based on the same criteria used for mammals: cytological demonstration of inclusion bodies in monocytes, compatible clinical signs such as arthralgia, in-coordination and vomiting, and response to doxycycline therapy (Tarello, 2006c).
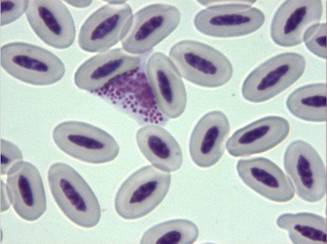
Fig. 16 - Ehrlichia spp. organisms in the cytoplasm of a monocyte (x100)
Arthralgia and neck pain are common clinical signs in affected mammals (Tarello, 2005). Fits of head’s tics or unvoluntary movements accounting for ‘neck pain’ or muscular spasm are occasionally see in falcons (see Movie n. 9):
Table 5 – Four cases of infction due to monocytic Ehrlichia spp. in falcons
N. Species, sex, age, weight Disease duration Clinical signs and (% monocytes affected) Clinical outcomes
1- Saker, F, 5 years, 1070 gr., 3 months Arthralgia, neck rigidity & tremors (17%) Complete clinical recovery
2- Saker, M, 1 year, 784 gr., 1 month Anorexia, lethargy, neck rigidity (22%) Complete clinical recovery
3- Saker, M, 1 year, 745 gr., 3 days Anorexia, vomiting, arthralgia (25%) Complete clinical recovery
4 -Saker, F, 1 year, 1100 gr., 2 weeks Anorexia, nose bleeding, dyspnoea (12%) Complete clinical recovery
Genus Ehrlichia contains obligate intracellular gram positive bacteria belonging to the family Rickettsiaceae, characterized by their unique tropism for circulating leucocytes and platelets. Several Ehrlichia spp. can be transmitted to a variety of hosts in nature, including dogs, horses, cats, roe-deers, boars, bears, mooses, chamois, birds and human beings (Tarello, 2006c). Usually, Ixodes ticks are the vectors. Definitive diagnosis is made by cytological demonstration of morulae (i.e., inclusions formed by clusters of rickettsiae) in leukocytes or by PCR.
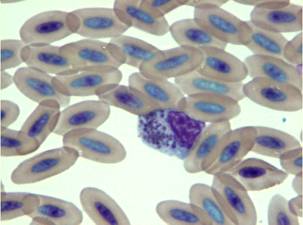
Fig. 17 – Ehrlichia inclusion bodies in a falcon’s monocyte
The predominant signs of ehrlichiosis are anorexia, lethargy, arthralgia, neck rigidity, lymphadenomegaly, weight loss, incoordination and pale mucous membranes. Stomatitis, conjunctivitis and opportunistic fungal infections have been also recorded in cats (Tarello, 2005).
Wild birds carrying infected ticks may be reservoir hosts for Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME) and for Anaplasma phagocytophilum, the zoonotic agent of granulocytic ehrlichiosis.
Therapy in falcons is based on oral administration of doxycycline mixed with the meat at 25 mg/kg once a day for 20 days. Clinical remission from signs highly evocative of ehrlichiosis, such as arthralgia, neck rigidity (altered posture of the neck), lethargy, in-coordination, tachypnea and bleeding from the nose, is observed at the end of therapy in association with a sharply decreased number infected cells or disappearance of mononuclear inclusions in falcons (Tarello, 2006c).
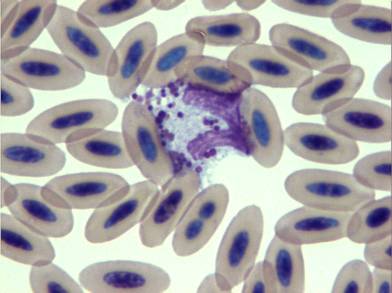
Fig. 18 – Ehrlichia spp. inclusion bodies in a monocyte of a falcon showing compatible signs
Normal falcon monocytes do not possess granules (Wernery et al., 2004). Occasionally, cells with cytoplasmatic granules have been seen in the peripheral blood of falcons and have been defined as ‘toxic granulations’ associated with underlying systemic illness (Wernery et al., 2004). This definition is compatible with the diagnosis of ehrlichiosis. The response to long-term doxycycline treatment is consistent with the cytological diagnosis of ehrlichiosis and is compatible with the suggested criteria for its diagnosis.
The accurate diagnosis of blood parasites is time-consuming and requires good quality Diff-Quick-stained thin blood smears (Peirce, 2003).
Infection with haematozoa may last for years in their avian hosts (Atkinson and van Riper, 1991). Many parasites cause seasonal relapses, often stress-induced or associated with increase of vector availability (Peirce, 2003). This is obviously not without immunological and clinical consequences (Remple, 2004). Accordingly, detection of pathogenic effects, mortality and response to specific therapies in infections due to intracellular blood parasites in falcons should not be controversial.
REFERENCES
ATKINSON, C.T. & van RIPER, C. III (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon and Haemoproteus. In: J.E. Loye, M. Zuk (ed.): Bird-parasite interactions, ecology, evolution and behaviour. Orford University Press, New York, 19-48.
BENNETT, G.F., PEIRCE, M.A. & ASHFORD, R.W. (1993) Avian haematozoa: mortality and pathogenicity. Journal of Natural History 27, 993-1001.
BENNETT, G.F., PEIRCE, M.A. & EARLE, R.A. (1994) An annotated checklist of the valid avian species of Haemoproteus, Leucocytozoon (Apicomplexa: Haemosporida) and Hepatozoon (Apicomplexa: Haemogregarinidae). Systematic Parasitology 29, 61-73.
GARVIN, M.C., HOMER, B.L. & GREINER, E.C. (2003) Pathogenicity of Haemoproteus danilewskyi, Kruse, 1890, in blue jays (Cyanocitta cristata). Journal of Wildlife Diseases 39, 161-9.
HEIDENREICH, M. (1997) Parasitic diseases. In: Birds of prey, medicine and management. Blackwell Science, Oxford, UK, 131-152.
KIRKPATRICK, C.E. & LAUER, D.M. (1985) Hematozoa of raptors from southern New Jersey and adjacent areas. Journal of Wildlife Diseases 21, 1-6.
KRONE, O., PRIEMER, J., STRICH, J., SOMMER, P., LANGGEMACH, T. & LESSOW, O. (2001) Haemosporida of birds of prey and owls from Germany. Acta Protozoologica 40, 281-289.
LIERZ, M., GOBEL, T. & SCHUSTER, R. (2002) Occurrence of parasites in indigenous birds of prey and owls. Berl. Munch. Tierarztl. Wochenschr. 115, 43-52.
LIERZ, M. & KRONE, O. (2002) Blood parasites in falcons in the United Arab Emirates. Proceedings of XIII DVG-Conference about avian diseases, Munich, Germany, February 2002, 121-124.
MUNOZ, E., FERRER, D., MOLINA, R. & ADLARD, R.D. (1999) Prevalence of haematozoa in birds of prey in Catalonia north-east Spain. Veterinary Record 144, 632-636.
OLSEN, G.H. & GAUNT, S.D. (1985) Effects of hemoprotozoal infections on rehabilitation of wild raptors. Journal of American Veterinary Medical Association 187, 1204-1205.
PEIRCE M.A. and BEVAN B.J. (1977) Blood parasite of imported psittacine birds. Veterinary Record 100, 282-3.
PEIRCE, M.A. (2000) A taxonomic review of avian piroplasms of the genus Babesia Starcovici, 1893 (Apicomplexa: Piroplasmorida: Babesiidae). Journal of Natural History 34, 317-332.
PEIRCE, M.A. (2003) Hematozoa. In: Avian Medicine. 2nd edn. Ed J. Samour. Mosby Ed, Elsevier Science, Edimburgh, 245-252.
REMPLE, J.D. (2004) Intracellular hematozoa of raptors: a review and update. Journal of Avian Medicine and Surgery 18, 75-88.
SAMOUR, J.H. & PEIRCE, M.A. (1996) Babesia shortti infection in a Saker falcon (Falco cherrung). Veterinary Record 139, 167-168.
SAMOUR, H.J. (2005) Therapeutic management of Babesia shortti infection in a Peregrine falcon (Falco peregrinus). Journal of Avian Medicine and Surgery 19, 294-296.
REGGIARDO, C. (1997) Haemoproteus spp. in an adult died dove. Arizona Veterinary Diagnostic Laboratory Quarterly 2, 4.
TARELLO W. (2001) Aegyptianella-like organisms and microfilariae in a severely diseased Bittern (Botaurus stellaris stellaris). Revue Med. Vet., 152, 189-193.
TARELLO, W. & RICCIERI, N. (2003) Aegyptianella-like inclusion bodies in two birds of prey from central Italy. Revue de Medecine Veterinaire 154, 715-717.
TARELLO, W. (2005) Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Veterinary Record 156, 772-774.
TARELLO, W. (2006a) Effective imidocarb dipropionate therapy for Babesia shortti in falcons. Veterinary Record 158, 239-40.
TARELLO, W. (2006b) Aegyptianellosis in falcons from Kuwait. Revue de Medecine Veterinaire 157, 266-269.
TARELLO, W. (2006c) Microscopic and clinical evidence for Ehrlichia spp. infection in Saker falcons (Falco cherrug). Revue de Medecine Veterinaire, 157, 203-204.
TARELLO, W. (2006d) Leucocytozoon toddi in falcons from Kuwait: epidemiology, clinical signs and response to melarsomine. Parasite 13, 179.
TARELLO, W. (2007) Clinical signs and response to primaquine in falcons with Haemoproteus tinnunculi infection Veterinary Record 161, 204-205.
WERNERY, R., WERNERY, U., KINNE, J. & SAMOUR, J. (2004) Colour Atlas of Falcon Medicine. Schlutersche Verlagsgesellschaft mBH & Co., Hannover, 2004, 93-96.
ZIMAN, M., COLAGROSS-SCHOUTEN, A., GRIFFEY, S. & STEDMAN, B. (2004) Haemoproteus spp. and Leucocytozoon spp. in a captive raptor population. Journal of Wildlife Diseases 40, 137-40.
First Published December 2007
Copyright Priory Lodge Education Limited 2007
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


