RHABDOMYOSARCOMA IN A DOG: CLINICAL AND PATHOLOGICAL FINDINGS
Sevil Atalay VURAL, Mehmet SAĞLAM, Mehmet HALIGUR, Hikmet KELEŞ
Rhabdomyosarcomas (RMS) are the most common neoplasm arising skeletal muscle, but it still represents less than 1% of all spontaneous neoplasms in domestic animals (Hulland, 1990). On the other hand RMS are reported about 50% all soft tissue neoplasms in child (Harms, 1995). There is no apparent sex, breed and regional predispositions.
In the case; clinical, patomorphological and immunohistological findings of rhabdomyosarcoma in a 6-year-old Kangal Dog have been described.
A 6-year old, male Kangal Dog with a growing mass noticed 2 months ago on right cubiti joint constituted the material.
General anesthesia was maintained by ketamine HCl 10% (Alfamine¨ , 10 mg/ml, Alfasan) following premedication with xylazine 2% (Alfazyne¨ , 20 mg/ml, Alfasan). The region was prepared for the operation on routine basis. The mass was removed through a blunt dissection after an elliptical skin incision on its base. The region was closed by means of suturing the skin and under tissues individually. Rifampicine¨ (250 mg) was administered locally. The wound was protected. Parenteral antibiotherapy was followed for 5 days postoperatively; skin sutures and the bandage were removed on the 10th day. The case was followed for 12 months and no recurrence was observed.
The mass was of 6x4x2.5 cm size and 30 g weight; it was quite stiff and presented a yellowish and moist cut section. It was fixed with 10% formaline and processed according to routine procedures, and stained with Hematoxylin and Eosine (HE), MassonŐs trichrome stain, and immunoperoxidase method (Avidin-biotin complex peroxidase-ABC-P). As for ABC-P method, anti-alfa actin, anti-desmin and anti-vimentin (1:200 dilution) were used as primary antibody.
Microscopically, numerous neoplastic cells of different sizes and staining characteristics and oval nuclei, proceeding to various directions in sheaves were observed (Fig. 1). Mitotic figures and giant cells were noticed among these cells. In MassonŐs trichrome staining, the nuclei were detected to be basophilic, and the cytoplasms and muscle fibers were in pink color (Fig. 2). In immunoperoxidase staining, the cells were given positive reaction with anti-actin and vimentin sera, diffuse and brownish color (Fig. 3-4). The mass was diagnosed as rhabdomyosarcoma, according to the results obtained.
Canine RMS have been reported as occurring in the pharynx, larynx, gingiva, urethra, bladder, cardiac muscle, greater, omentum, trachea and tongue (Hulland, 1990). Primary RMS of limb muscles were seen rare (Ginel et al, 2002; Hulland, 1990) The neoplasm can occur in almost all ages, however it is frequent at around 2-3 years. As for the case, RMS was encountered in a 6-year-old dog and it was diagnosed because of rarely seen on limb muscle.
The neoplasm causes clinical symptoms such as lameness and irregular swellings on the legs (Hulland, 1990) and causes disorders in the tissues or organs that it metastases to (lymph nodes, lungs, heart, spleen, adrenal glands, kidneys) (Worley and Gorham, 1954). Similar swellings, pain and lameness were observed in our case however, no metastasis occurred during the follow-up period.
Rhabdomyosarcomas are classified histomorphologically into 4 categories: alveolar, embriyonal, botryoid and pleomorphic type (Hulland, 1990). In the case, histomorphologically the neoplasm was diagnosed pleomorphic type.
In some of the rhabdomyosarcomas, diagnosis could be difficult due to concincing striations and various histomorphological appearances (Hulland, 1990) particularly in the lack of an evident histomorphological finding indicating rhabdomyoblastic differentiation (De Las Mulas et al, 1992). Since that, different cytochemical stains are utilized which were thought to be insufficient for diagnosis but used for showing cross or longitudinal striations or to detect mesenchymal tissues ((De Las Mulas et al, 1992; Meyvisch et al,1977). In the case, MassonŐs trichrome staining was used to show striated muscles. Furthermore, immunohistochemical examinations have started to be utilized recently, in order to show cellular myoglobulin of intermediate filament proteins (De Las Mulas et al, 1992; Brooks, 1982). Although Hulland (1990) mentioned that this method would not be applicable in animal tissues yet, it has been experimented in animals many times (De Las Mulas et al, 1992; Truong et al, 1990; Langbein et al, 1989; Andreasen et al, 1988; Madewell et al, 1988; Takahashi et al, 1988). Likewise, anti-actin, anti-desmin and anti-vimentin sera were utilized as diagnostic markers in immunperoxidase staining in the case and striated muscles reacting positive with anti-actin and anti-vimentin sera were detected. The neoplastic cells were given negative result with anti-desmin sera. On the other hand the neoplasm was thought primitive, which lacked desmin similar to Molenaar et al (1985) and it was evaluated because of rarity seen.
Literatures
1. Andreasen, CB., White, MR., Swayne, DE. and Graves, GN. (1988). Desmin as a Marker for Canine Botryoid Rhabdomyosarcomas. J Comp Pathol. 98(1), 23-29.
2. Brooks, JJ. (1982). Immunohistochemistry of Soft Tissue Tumors. Myoglobin as a Tumor Marker for Rhabdomyosarcoma. Cancer. 50(9), 1757-1763.
3. De Las Mulas, M., Vos, JH. and Van Mil, FN. (1992). Desmin and Vimentin Immunocharacterization of Feline Muscle Tumors. Vet PathoI. 29(3), 260-262.
4. Ginel, PJ., De las Mulas, M.J., Lucena, R., Millan, Y. and Novales, M. (2002). Skeletal Muscle Rhabdomyosarcomas in a Dog. Vet Rec. 151, 736-738.
5. Harms, D (1995): Soft Tissue Sarcomas in the Kiel Pediatric Tumor Registry. Curr Top Pathol. 89, 31-45.
6. Hulland, TJ. (1990). Tumors of the Muscle. In: Tumors in Domestic Animals, 3rd ed., Ed, Moulton JE, pp.88-101. University of California Press, Berkeley.
7. Langbein, L., Kosmehl, H., Kiss, F., Katenkamp, D. and Neupert, G. (1989). Cytokeratin Expression in Experimental Murine Rhabdomyosarcomas. Intermediate Filament Pattern in Original Tumors, Allotransplants, Cell Culture and Re-established Tumors from Cell Culture. Exp Pathol. 36, 23-26.
8. Madewell, B., Lund, J., Munn, R. and Pino, M. (1988). Canine Laryngeal Rhabdomyosarcoma: an Immunohistochemical and Electron Microscopy Study. Nippon Juigaku Zasshi. 50, 1079-1084.
9. Meyvisch, C., Thoonen, H. and Hoorens, J. (1977). The Ultrastructure of Rhabdomyosarcoma in a Dog. Zentralbl Veterinarmed A. 24, 542-51.
10. Molenaar, WM., Oosterhuis, JW., Oosterhuis, AM. and Ramaekers, FC. (1985). Mesenchymal and Muscle-specific Intermediate Filaments (Vimentin and Desmin) in Relation to Differentiation in Childhood Rhabdomyosarcomas. Hum Pathol. 16, 838-843.
11. Takahashi, K., Maita, K., Shirasu, Y., Taniguchi, H. and Yoshikawa, Y. (1988). Anti-rat Myoglobin Antisera in the Immunocytochemical Diagnosis of Rhabdomyosarcomas of Rats. Vet Pathol. 25, 337-342.
12. Truong, LD., Rangdaeng, S., Cagle, P., Ro, JY., Hawkins, H. and Font, RL. (1990). The Diagnostic Utility of Desmin. A Study of 584 Cases and Review of the Literature. Am J Clin Pathol. 93, 305-314.
13. Worley, G. and Gorham, JR. (1954). The Comparative Pathology of Rhabdomyosarcoma with a Report of a Case in a Dog. Am J Pathol. 30, 837-849.
FIGURES:
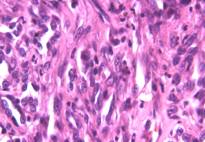
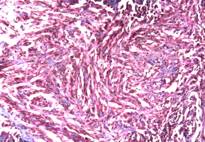
Figure 1 Figure 2
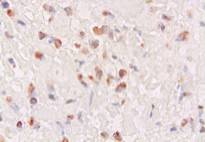
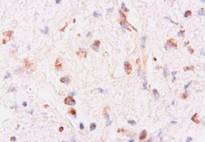
Figure 3 Figure 4
Figure Legends:
Figure 1: Appearance of neoplastic cells, HE, x400.
Figure 2: Appearance of neoplastic cells, MassonŐs trichrome stain, x100.
Figure 3: Positive immunoreactivity with anti-actin sera, ABC-P stain, x400.
Figure 4: Positive immunoreactivity with anti-vimentin sera , ABC-P stain, x400.
Authors
Sevil Atalay Vural, PhD, Department of Pathology, Ankara University, Faculty of Veterinary Medicine, Ankara, TURKEY
Mehmet Saglam, PhD, Department of Surgery, Ankara University, Faculty of Veterinary Medicine, Ankara, TURKEY
Mehmet Haligur, PhD, Department of Pathology, University of Akdeniz, Faculty of Veterinary Medicine, Burdur, TURKEY
Hikmet Keles, PhD, Department of Pathology, University of Afyon Kocatepe, Faculty of Veterinary Medicine, Afyon, TURKEY
Correspondence address: Sevil Atalay Vural, PhD, Department of Pathology, Ankara University, Faculty of Veterinary Medicine, Ankara, TURKEY
Fax: +90-312-3164472
Phone: +90-312-3170315 / 276
E-mail: svural@veterinary.ankara.edu.tr
sevilvural@yahoo.com
First Published: January 2005
All pages copyright ©Priory Lodge Education Ltd 1994-2005.