Browse through our Journals...
Immunohistochemical Localization of TNFa and NOS in the Equine Corpus Luteum
Omar M. Al-zi’abi1, Hamish M. Fraser2 and Elaine Watson3
1Department of anatomy, Faculty of Veterinary Medicine, Hama –Syria, 2MRC Human Reproductive Sciences Unit, 37 Chalmers Street, Edinburgh EH3 9ET, UK and 3Department of Veterinary Clinical Studies, University of Edinburgh, Easter Bush, Midlothian EH25 9RG, UK.
Keywords: Equine, Corpus luteum, Luteolysis and Cytokines
Abstract
In mares, little information is available on the role of TNFα and NO during corpus luteum development and demise. Immuno-localization was carried out on corpora lutea collected from mares in the early luteal phase (days 3–4; n= 4); mid-luteal phase (day 10; n= 5); early regression (day 14; n= 4); late regression (day 17; n= 4) and 12 and 36 h (n= 3 per group) after PGF2a administration on day 10. TNFα immunostaining was found in the cytoplasm of luteal and non-luteal cells, including neutrophils.
The percentage area of TNFa immunostaining was high during the early luteal phase then decreased significantly (p < 0.01) at mid-luteal phase and early regression and increased during late regression and 36 h following PGF2a (p < 0.01).
iNOS immunostaining was seen in the cytoplasm of luteal cells during the early and late luteal phase. The percentage area of iNOS immunostaining was significantly (p < 0.01) higher during regression in comparison with the early and mid-luteal phase.
eNOS immunostaining was limited to a few luteal cells and endothelial cells during early and mid-luteal phase and little immunostaining was found during natural and induced regression. The percentage area of eNOS immunostaining was significantly (p< 0.05) higher during early and mid-luteal phase. The overall results suggest that TNFa and NO help luteal development and regression.
Introduction
The equine corpus luteum (CL) is functional for about 14-15 days in non-fertile cycles (Hughes et al., 1975) and its regression is characterised by a decrease in progesterone production (functional regression) and cellular demise (structural regression) (Al-zi'abi et al., 2002). In a previous study we reported the involvement of apoptosis in both natural and induced luteolysis in the equine CL (Al-zi'abi et al., 2002).
Apoptosis is regulated by molecules, such as cytokines, growth factors, peptide hormones, reactive oxygen species (ROS) and steroids. Cytokines, tumour suppressor genes, death genes and survival genes have been found to play a role in the fate of human (Matsubara et al., 2000) and bovine luteal cells (Okuda and Sakumoto 2003).
Moreover, recent data show that TNFa and nitric oxide are involved in luteal regression. TNFa is a protein produced by macrophages, promoting luteal regression (Bagavandoss et al., 1990). In fact, luteal cells have been reported to contain immunoreactivity for TNFa (Bagavandoss et al., 1990). TNFa inhibits gonadotrophin-supported progesterone production in luteal cells of many species including murine (Adashi et al., 1990), porcine (Pitzel et al., 1993), and bovine (Benyo and Pate, 1992) and it is thought to stimulate PGF2a synthesis by bovine luteal cells (Bagavandoss et al., 1990).
Nitric oxide (NO) is a powerful vasodilator and mediator of macrophage cytotoxicity and apoptosis (Lowenstein and Snyder, 1992). Two isoforms, endothelial NOS (eNOS) and inducible NOS (iNOS) have been detected in the ovarian tissue of rat and mouse (Van Voorhis et al., 1995; Jablonka-Shariff and Olson, 1997). Human luteinised granulosa cells showed eNOS mRNA expression (Van Voorhis et al., 1994).
NO induces apoptosis in vitro in human luteal cells (Vega et al., 2000) and it seems to be a luteolytic factor in the human CL (Friden et al., 2000).
TNFa and NO (Friden et al., 2000) are thought to be involved in luteolysis in many species by inducing apoptosis in vitro. However, little information is available on the exact factors involved in apoptosis in the equine CL. We investigated the immunolocalisation of TNFa, eNOS and iNOS, throughout the cycle and following PGF2a injection, to explore the role of these factors in luteal regression in equine CL.
Materials and Methods
Animals and tissue collection
The ovaries of mixed-breed pony mares, aged 4-12 years and weighing 250-450 kg, were examined daily during oestrus by trans-rectal ultrasonography to determine the day of ovulation (day 0). Corpora lutea were obtained in the early luteal phase (days 3–4; n = 4), mid-luteal phase (day 10; n= 5), early regression (day 14; n= 4), late regression (day 17; n= 4) and at 12 and 36 h (n= 3 each) after IM administration of the PGF2-α analogue, cloprostenol (Estrumate, 263 µg for 500,Schering-Plough Animal Health, Middlesex) on day 10 of the oestrous cycle. The corpus luteum was enucleated from the ovary and dissected free of connective tissue. Tissue samples were fixed in 10% (v/v) neutral phosphate buffered formalin (pH 7.0) for 24 h and then embedded in paraffin wax. Serial sections of 4 mm were mounted on to glass microscope slides coated with poly-L-lysine (Sigma, Poole, USA). This study was performed under the approval of the University of Edinburgh Ethical Review Committee and the project licence obtained under the Home Office Animals (Scientific Procedures) Act 1986.
Immunocytochemistry
Sections of formalin fixed tissue (4 mm) were mounted on slides coated with BioBond (British Biocell Int, Cardiff), deparaffinized and rehydrated. Endogenous peroxide activity was blocked by immersion in 3% (v/v) H2O2 in methanol for 30 min. After two washes of 5 min each in 0.01 mol PBS (pH 7.4), sections were incubated for 120 min at room temperature in a humidified chamber or overnight at 4 °C with goat anti-TNFa (Santa Crus Biotechnology) diluted to 1:250 in PBS. Four eNOS and iNOS sections were treated with citrate buffer and exposed to three cycles of 5 min each in the microwave, then incubated for 120 min at room temperature with rabbit anti-human eNOS and rabbit anti-mouse iNOS (BD Transduction Laboratories, Lexington, USA) diluted to 1:500 in PBS. Negative controls were incubated with PBS containing normal goat IgG instead of the primary antibody. ABC method was used and sections were visualized with 0.05% (w/v) 3,3-diaminobenzidine containing 0.01% (v/v) H2O2 (Sigma) and counterstained with haematoxylin.
The percentage area of TNFa, iNOs and eNOS immunostaining
Quantimet image system was used to estimate the percentage area of immunostaining, thus the area of the immunostaining stain divided by the total area measured x100.
The results were expressed as percentage mean ± SEM per unit area.
Results
TNFa immunostaining
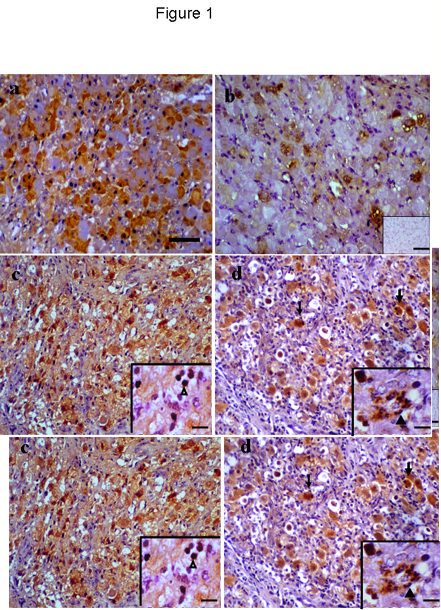
Positive immunostaining was found in the cytoplasm of luteal cells in all sections examined throughout the cycle. Non-luteal cells, including neutrophils were also immunostained during regression. No positive staining was observed in negative control sections (Fig 1b, inset) and no apparent staining was detected in endothelial cells. Some growing luteal cells exhibited immunoreactivity during the early luteal phase (Fig 1a) and by the mid-luteal phase (Fig 1b) a few cells were immunostained.
Pale immunostaining was found in a few luteal cells during early regression; by late regression most of the luteal cells showed immunostaining (Fig 1c) as well as some non-luteal cells (Fig 1c, inset). Twelve and 36 h following PGF2a-induced regression some luteal cells showed strong immunostaining including those that underwent apoptosis (Fig 1d) as well as some neutrophils (Fig 1d, inset).
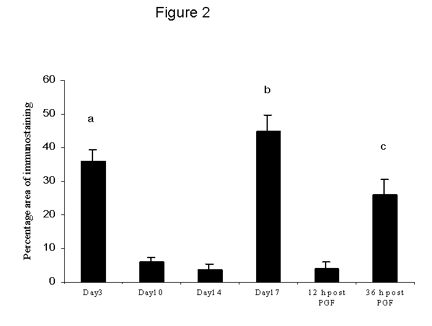
The percentage area of TNFa immunostaining was high during the early luteal phase then decreased significantly (p < 0.01) at mid-luteal phase and early regression (Fig 2). By late regression and 36 h following PGF2a there was significant increase in TNFa immunostaining (p < 0.01) compared with early regression and 12 h following PGF2a.
NOS immunostaining
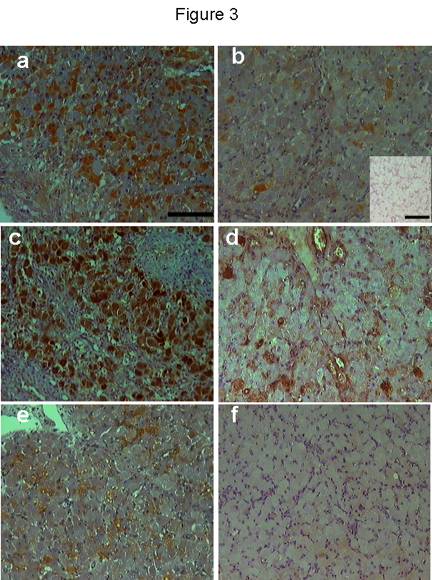
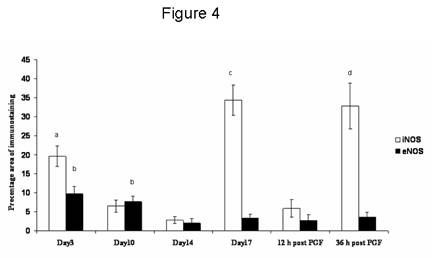
iNOS immunostaining was seen in the cytoplasm of luteal cells in all sections examined throughout the cycle. No positive staining was observed in negative control sections (Fig 3b, inset). Positive staining was found in some luteal cells during the early luteal phase (Fig 3a) and a few mature luteal cells during the mid-luteal phase (Fig 3b). During natural and induced regression, luteal cells were densely immunostained (Fig 3c) showing a percentage area (Fig 4) significantly (p < 0.01) higher compared with the early and mid-luteal phase. eNOS immunostaining was limited to a few luteal cells and endothelial cells during early (Fig 3d) and mid-luteal phase (Fig 3e). Little immunostaining was found during natural and induced regression (Fig 3f). The percentage area of eNOS immunostaining (Fig 4) was significantly (p < 0.05) higher during early and mid-luteal phase in comparison with regression phase.
Discussion
TNFa and iNOS intense immunostaining associated with structural luteolysis, suggest their involvement in cell death. The pattern of eNOS immunostaining during early and mid-luteal phase seems to support the development and angiogenesis of CL.
This seems to be the first report on the immunolocalisation of TNFa in the equine CL. Luteal and non-luteal cells, such as neutrophils, were immunostained for TNFa. Previous studies indicate luteal cells and macrophages as the main sources of TNFa in the human (Roby et al., 1990), bovine (Roby and Terranova, 1989; Zolti et al., 1990) and rabbit CL (Bagavandoss et al., 1990). Neutrophils are the main source of many cytokines including TNFa, and have shown immunostaining for TNFa in many other tissues including endometrium (Von Wolff et al., 1999) and blood (Djeu et al., 1990).
Changes in TNFa immunostaining were observed throughout the luteal phase. This may suggest the involvement of TNFa in CL development and maintenance. Moreover, the presence of TNFa in luteal cells possibly indicates a paracrine and/or autocrine luteotrophic role for TNFa (Adashi et al., 1990). Previous studies assumed that TNFa is one of the factors mediating luteotrophic function (Kirsch et al., 1983) and stimulating luteal progesterone secretion (Terranova et al., 1991). Increase in granulosa proliferating pig cells treated with TNFa was noticed in vitro (Prange-Kiel et al., 2001). In the present study, however, the intense immunostaining observed during late regression phase suggests a possible role for TNFa in luteal regression.
Different hypotheses, including the stimulatory effect on PGF2a release in vitro (Zolti et al., 1990), inhibition of LH action (Benyo et al., 1992), induction of apoptosis and of structural regression (Prange-Kiel et al., 2001, Petroff et al., 2001) have been suggested.
The peak increase in TNFa immunostaining at late regression (day 17) does not support any of these hypotheses because structural luteal regression was evident in early regression (day 14). During late regression and 36 h following PGF2a injection, luteal cells with pyknotic appearance showed intense immunostaining for TNFa. These findings suggest a direct role for TNFa in luteal cell demise through apoptosis pathway.
eNOS was restricted to the early and mid-luteal phase, while some endothelial and a few luteal cells were immunostained, not in contrast with previous studies showing immunostaining of eNOS in endothelial and luteal cells from rodents (Van Voorhis et al., 1995; Jablonka-Shariff and Olson, 1997) and humans (Van Voorhis et al., 1994; Vega et al., 1998; Friden et al., 2000). This may indicate that eNOS helps the CL development and angiogenesis. Actually, angiogenesis is evident in the in equine CL in early and mid-luteal phase (Al-zi'abi et al., 2003). NO is known to induce angiogenesis and vascular permeability (Motta et al., 1999) whereas NO inhibition reduces such effects (Papapetropoulos et al., 1997). eNOS apparently enhance angiogenesis and cell proliferation (Jadeski et al., 2000) and it is considered an anti-apoptotic factor (Dimmeler and Zeiher, 1997). On the other hand, iNOS detection was limited to the luteal cells during the luteal phase and following PGF2a.-induced regression, in accord with previous findings (Friden et al., 2005). During natural regression and induced regression, intense immunostaining of iNOS was evident, suggesting that this isoform predisposes to luteal regression and cell death. iNOS is thought to be a pro-apoptotic factor (Dimmeler and Zeiher, 1997) and a luteolytic factor in rat and human luteal cells (Vega et al., 2000; Friden et al., 2000; Motta et al., 2001). Intra-luteal injections of the NO blocker (Nomega-Nitro-L-Arginine Methyl Ester (L-NAME), an inhibitor of nitric oxide synthase (NOS), showed increased production of progesterone during CL regression in cows, indirectly supporting a luteolytic action of NO in the bovine CL (Jaroszewski and Hansel, 2000). Moreover, PGF2a-induced regression can up-regulate NOS activity and consequently the production of NO which acutely inhibits progesterone release, strongly suggesting that NO participates in functional regression by inhibiting steroidogenesis in cows (Jaroszewski and Hansel, 2000).
In summary, our findings show for the first time the immuno-localization of TNFa and NO in the equine CL, thus implicating both in luteal development and regression.
Acknowledgement
This work was carried out at the Department of Veterinary Clinical Studies, University of Edinburgh, and was funded by AL-ba'ath University- Syria as Ph.D fellowship for the author.
References
Adashi EY, Resnick CE, Packma JN, Hurwitz A & Payne DW (1990). Cytokine-mediated regulation of ovarian function:TNFa inhibits gonadtropin-supported progesterone accumulation by differentiating and luteinised granulosa cells. American Journal of Obstetrics and Gynaecology 162, 889-899.
AL-zi’abi MO, Fraser H M & Watson E D (2003). Angiogenesis and cell proliferation in the equine corpus luteum. Reproduction 125, 259-270.
AL-zi’abi MO, Fraser H M and Watson E D (2002). Cell death during natural and induced luteolysis in the mare. Reproduction 123, 67-77.
Bagavandoss P, Kunkel SL, Wiggins RC, and Keyes PL (1989). Tumor necrosis factor production and localization of macrophages and T lymphocytes in the rabbit corpus luteum. Endocrinology 122, 1185-1187.
Bagavandoss P, Wiggins RC, Kunkel SL, Remwick DG & Keyes PL (1990). Tumor necrosis factor production and accumulation of inflammatory cells in the corpus luteum of pseudopregnancy and pregnancy in rabbits. Biology of Reproduction 42, 367-376.
Benyo DF & Pate JL (1992). TNFa alters bovine luteal cell synthetic capacity and viability. Endocrinology 130, 854-860.
Dimmeler S and Zeiher AM (1997). Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide 1, 275-281.
Djeu JY, Serbousek D & Blanchard DK (1990). Release of tumour necrosis factor by human polymorphonuclear leukocytes. Blood 76, 1405-1409.
Friden EB, Runessen E & Hahlin M (2000). Evidence for nitric oxide acting as a luteolytic factor in the human corpus luteum. Molecular Humam Reproduction 6, 397-403.
Hughes JP, Stabenfeldt GH & Evans JW (1975). The oestrous cycle of the mare. J Reprod Fertil Suppl 23, 161-6.
Jablonka-Shariff A & Olson LM (1997). Hormonal regulation of nitric oxide synthases and their cell-specific expression during follicular development in the rat ovary. Endocrinology 138, 460-468.
Jadeski LC, Hum KO, Chakraborty C and Lala PK (2000). Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. International Journal of Cancer 86, 30-39.
Jaroszewski JJ, Hansel W (2000). Intraluteal administration of a nitric oxide synthase blocker stimulates progesterone and oxytocin secretion and prolongs the life span of the bovine corpus luteum. Proceeding Society of Experimental Biology and medicine 224, 50-55.
Kirsch TM, Vogel RL & Flickinger GL (1983). Macrophages: a source of luteotropic cybernins. Endocrinology 113, 1910-1912.
Lawler DF, Hopkins J & Watson ED (1999). Immune cell populations in the equine corpus luteum throughout the oestrous cycle and early pregnancy: an immunohistochemical and flow cytometric study. Journal of Reproduction and Fertility 117, 281-290.
Lowenstein C and Snynder SH (1992). Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524-526.
Matsubara H, Ikuta K, Ozaki Y, Suzuki Y, Suzuki N, Sato T & Suzumori K (2000). Gonadotropins and cytokines affect luteal function through control of apoptosis in human luteinized granulosa cell. Journal of Clinical Endocrinology and Metabolism 85, 1620-6.
Motta AB, Estevez A, Tognetti T, Gimeno MAF and Franchi AM (2001). Dual effects of nitric oxide in functional and regressing rat corpus luteum. Molecular Human Reproduction, 7, 43-47.
Okuda K & Sakumoto R (2003). Multiple roles of TNF super family members in corpus luteum function. Reprod Biol Endocrinol 10, 1:95.
Papapetropoulos A, Desai KM, Rudic RD, Mayer B, Zhang R, Ruiz-Torres MP, Garcia-Cardena G, Madri JA and Sessa WC (1997). Nitric oxide synthase inhibitors attenuate transforming-growth-factor-beta 1-stimulated capillary organization in vitro. American Journal of Pathology 150, 1835-1844.
Petroff MG, Petroff BK & Pate JL (2001). Mechanisms of cytokine-induced death of cultured bovine luteal cells. Reproduction121, 753-760.
Pitzel L, Jarry H & Wuttke (1993). Effects and interactions of prostaglandin PGF2a oxytocin, and cytokines on steroidogenesis of porcine luteal cells. Endocrinology, 132, 751-756.
Prange-Kiel J, Kreutzkamm C, Wehrenberg U and Rune GM (2001). Role of tumour necrosis factor in preovulatory follicles of swine. Biology of Reproduction, 65, 928-935.
Roby KF & Terranova PF (1989). Localization of tumour necrosis factor TNFa in rat and bovine ovary using immunocytochemistry and cell blot: evidence for granulosal production. Hirshfield AN, ed. Growth Factors of the ovary. New York Press: Plenum Press, pp 273-278.
Shibata M, Parfenova H, Zuckerman SL & Leffler CW (1996). TNFa induces pial arteriolar dilation in newborn pigs. Brain Research Bull 39, 241-247.
Terranova PF, Roby KF, Sancho-Tello M, Weed J and Lyles R (1991). Tumor necrosis factor-?: localization and actions within the preovulatory follicle. In: Schomberg D, ed. Growth factors in Reproduction. New York: Springer Verlag, pp. 63-67.
Van Voorhis BJ, Dunn MS, Snyder GD & Weiner CP (1994). Nitric oxide: an autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology 135, 1799-1806.
Van Voorhis BJ, Moore K, Strijbos PJ, Nelson S, Baylis SA, Grzybicki D & Weiner CP (1995). Expression and localization of inducible and endothelial nitric oxide synthase in the rat ovary. Effects of gonadotrophin stimulation in vivo. Journal of Clinical Investigation 96, 2719-2726.
Vega M, Urrutia L, Iniguez G, Gabler F, Deveto L & Johnson, MC (2000). Nitric oxide induces apoptosis in the human corpus luteum in vitro. Molecular Human Reproduction 6, 681-687.
Von Wolff M, Classen-Linke I, Heid D, Krusche CA, Beier-Hellwig K, Karl C & Beier HM (1999). Tumour necrosis factor-alpha (TNF-alpha) in human endometrium and uterine secretion: an evaluation by immunohistochemistry, ELISA and semiquantitative RT-PCR. Molecular Human Reproduction 5, 146-152.
Zolti M, Meirom R, Shemesh M, Wollach D, Mashiach S, Shore L & Rafael ZB (1990) Granulosa cells as a source and target organ for tumor necrosis factor-alpha. Federation of American Society of Experimental Biology Journal 261, 253-255.
Figure legends
Figure1. TNFa immunostaining during luteal phase and following PGF2a-induced regression. a) some luteal cells showed cytoplasmic immunostaining during early luteal phase. b) by mid-luteal phase few luteal cells showed immunopositive, negative control (inset) free of any immunostaining. c-d) late luteal regression and 36 h post PGF2a injections showing immunopositive luteal cells, luteal cells with apoptotic appearance showed strong positive staining (thin black arrow). Immunostaining for TNFa were found in non-luteal cells probably lymphocytes and macrophages (arrowhead, c: inset) and neutrophils (arrowhead, d: inset) after PGF2a-induced regression. Scale bar represents 40 mm and 5 mm for the insets.
Figure 2. The percentage area of TNFa immunostaining throughout the cycle and after PGF2a administration. Data are expressed as percentage (mean ± SEM).
a: significantly (p < 0.01) higher mid-luteal phase.
b: significantly (p < 0.01) higher than mid-luteal phase and early regression.
c: significantly (p < 0.05) higher than mid-luteal phase and 12 h following PGF2a.
Figure 3. iNOS immunostaining during early luteal phase (a), mid-luteal phase (b), 36h following PGF2ainjection (c). Luteal cells showed immunostaining during early luteal phase while very few cells showed positive staining during mid-luteal phase. During natural and induced regression most of luteal cells showed strong immunostaining. No positive immunostaining was found in the negative control (b: inset). eNOS immunostaining was restricted to some endothelial cells and very few luteal cell during early (d) and mid-luteal phase (e). During early regression, pale immunostaining was found in few luteal cells (f). Scale bar represents 50 mm and 10 mm for the inset.
Figure 4. The percentage area of iNOS and eNOS immunostaining throughout the cycle and after PGF2a administration. Data are expressed as percentage (mean ± SEM).
a: significantly (p < 0.05) higher than mid-luteal phase and early regression
b: significantly (p < 0.05) higher than early and late regression.
c: significantly (p < 0.01) higher than early, mid-luteal phase and early regression.
d: significantly (p < 0.05) higher than mid-luteal phase and 12 h post PGF2a .
Copyright Priory Lodge Education Limited 2008
First published March 2008
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-


