Browse through our Journals...
Optic Lens Proteins of Sheep and Goat at Pre- and Postnatal Ages
M. Aminlaria*, S. Gholamib and E. Shahsavania
aDepartment of Biochemistry and bDepartment of Anatomical Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, 71345 Iran
Abstract
The purpose of this investigation was to study the optic lens proteins of sheep and goat at pre- and postnatal ages. Optic lenses were removed from embryos and slaughtered adult sheep and goats, homogenized and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoretic patterns of sheep and goat proteins were similar and the main protein bands were localized in the range of 17-34.4 kDa. The concentration of proteins was not affected by sex. In both species, a 19.3 kDa protein, identified as α-crystallin, was dominant and most concentrated. Differences between pre- and postnatal ages were the followings: a) an 18.4 kDa protein, assumed to be a γ-crystallin, appeared at the 3rd month of pregnancy and gradually increased in concentration until delivery remaining constant at all postnatal ages; b) the concentration of 23.6 and 34.4 kDa proteins gradually decreased with ageing; c) the 34.4 kDa protein appears at different fetal ages in sheep and goats and is absent in 4-year old animals, and c) several low molecular weight proteins (less than 17 kDa) appeared at postnatal ages, suggesting increased proteolysis in the adult stages.
Key words: Sheep; Goat, Crystallin; Optic lens; SDS-PAGE.
Introduction
The lens is a clear tissue suspended by zonular fibers between the acqueous humour and the anterior face of the vitreous body. The lens grows throughout life building up layer after layer of fibre cells around a central core. It never shed cells. So the cells at the centre of the lens are as old as the animal itself. Some proteins at the centre of the lens may be there for most of the lifetime of the animal. There are no other proteins which persist for so long and alterations occur as consequences of ageing and cataract (Harding and Dilley, 1976).
A third or more of the wet weight and almost all the dry material of the mammalian lens is protein. The water-soluble lens proteins in mammals account for approximately 90% of the proteins of the eye lens and are divided into three main classes: α-crystallin, β-crystallin and γ-crystallin on the basis of chemical characteristics. Each of these crystallins is a heterogeneous multimeric complex of subunits, with different molecular weights (De Jong, et al., 1994; Harding and Dilley, 1976; Wistow and Piatigorsky, 1988). The soluble lens proteins have been considered unique to the lens, although α-crystallin has been identified in non lenticular tissues as a small heat shock protein (Ingolia and Craig, 1982) and γ-crystallin has been found in the retina (Denis et al., 2003).
Calf α-, β- and γ-crystallins have been widely studied and used as model for investigations on the effect of different chemical and physical factors on lens proteins (Bjork 1961; van Kleef, et al., 1974). However, few reports on the biochemical properties of individual lens proteins of sheep (De Mate, et al., 1976; Ferguson and Koenig, 1972; Mehta and Lerman, 1971) and goat (Bera and Ghosh., 1999) are available. The purpose of this study was to identify and compare the lens proteins of sheep and goat at pre- and postnatal stages.
Materials and Methods
All chemicals were of analytical grade and were obtained from commercial sources. Lenses from sheep and goat were obtained from local slaughter houses. Age of sheep and goat fetuses were determined as described by Evans and Sack (1973). Lenses were stored frozen at -20 oC until use. Protein extracts were prepared by thawing and homogenizing the lenses in the presence of liquid nitrogen, using a hand homogenizer and suspending the homogenate in 0.025 M phosphate buffer, pH 7.0. In all cases studied, essentially a clear solution was obtained after homogenization. Protein content of extracts was determined by the method of Lowry, et al., (1951) using crystalline bovine serum albumin as standard. Protein extracts were subjected to sodium dodesyl sulfate poplyacrylamide gel electrophoresis (SDS-PAGE) according to the discontinuous buffer system of Laemmli (1970). Protein samples were prepared by diluting each sample in a sample buffer to give a final concentration of 1 mg protein /mL in 0.01M Tris-HCl, pH 6.8, 0.4% SDS, 10% glycerol and 0.04% bromophenol blue. The running gel with dimensions of 120 X 90 X 1 mm was made of 12.5% acrylamide or a 10-20% acrylamide gradient in 1.2 M Tris-HCl, pH 8.8 and 0.3% SDS. The stacking gel contained 3% acrylamide in 0.25 M Tris-HCl, pH 6.8 and 0.2%SDS. Samples were heated in boiling water for 10 minutes and 40 mL was applied to each slot. The electrode buffer compromised 0.025 M Tris-HCl, 0.192 M Glycine and 0.1%SDS at pH 8.16. Electrophoresis was performed at constant 25 mA and gels were fixed in a solution containing 45.4% methanol and 9.2% acetic acid. Gels were then stained with 0.25% Coomasie Brilliant Blue in 25% isoprepanol, 10% acetic acid and destained with a 5% acetic acid/7% methanol solution.
Results
SDD-PAGE identified multiple bands which corresponded with reported molecular weights of lens α-, β- and γ-crystallin subunits (Figs. 1-6; molecular weight size standards are on the left). Most proteins were separated in the range of ~17 to ~ 34 kDa. Eight main proteins had molecular weights of 17.0, 18.4, 19.6, 23.6, 26.0, 28.5, 32.3 and 34.4 kDa. In both species, the 19.3 kDa protein (α-crystallins subunit) was dominant and most concentrated. The SDS-PAGE pattern was essentially the same for both species and sexes with the following differences noted between pre- and postnatal ages (Fig.1,2,3,5): a) the 18.4 kDa protein appears at the 3rd month of pregnancy and gradually increases in concentration and remains constant at all postnatal ages; b) the concentration of the 23.6 and 34.4 kDa proteins decreases during adult life in both sheep and goats; c) the 34.4 kDa protein appears at different fetal ages in sheep (Fig. 3 ) and goats (Fig. 5) and is essentially absent in 4-year old animals (Figs. 1, 2, 4, 6); d) small molecular weight proteins (14 to 17 kDa) appear in adult lenses and are absent in fetuses or young animals.
Discussion
In this authors’ knowledge, this is the first report of pre-natal α-, β- and γ-crystallin originated from sheep and goats. Comparative studies of lens proteins at various stages of development are important because provide information on the biochemical functions of the crystallins during animal growth, considering also that some crystallins are similar or identical to metabolic enzymes (Wistow and Piatigorsky, 1987). The results of SDS-PAGE analysis confirm that there are few notable differences between pre- and post-natal optic lens proteins in sheep and goats.
Lens protein composition is altered by 3 main mechanisms: (1) changes in patterns of protein synthesis with time; (2) changes in lens proteins as a consequence of ageing; (3) changes due to lens protein breakdown of the fiber cells membranes (Harding and Dilley, 1976).
Examination of changes in lens proteins during pre- and post-natal development has been done in bovines (Francois and Rabaey, 1957; van Kamp et al., 1974) and humans (Maisel and Goodman, 1965) by comparing whole lenses of different ages.
In this study, a concentrated protein with molecular weight of 19.3 kDa was present in the lens of both species and corresponded with reported molecular weights of lens α–crystallin subunits. The α-crystallin of bovine (van Kleef et al., 1974 ; Stauffer et al., 1973), human (Clark et al., 1969), rabbit (Lien-The and Hoenders, 1974), pig, horse, dog, cat and rhesus monkey (de Jong et al., 1975; Denis et al., 2003), sheep (Ferguson and Koenig, 1972) and goat (Roy and Ghosh, 1991) are very similar and are made of four major subunits, A1, A2, B1 and B2, each having a molecular weight of approximately 20 kDa. The amino acid composition of A1 and A2 is very similar and differs considerably from that of B1 and B2, which are also similar to each other (Stauffer et al., 1973).
In adult animals, these subunits are subjected to proteolysis resulting in progressive degradation of these polypeptides to smaller fragments with molecular weight of 13-17 kDa (van Kamp et al., 1973). In this study, the SDS-PAGE revealed that several proteins in the range of 14 to 17 kDa are present at post-natal ages and absent during fetal life suggesting the action of proteases on the α- crystallin (Shearer et al., 1997).
Embryonic bovine α-crystallin has a similar amino acid composition to calf α-crystallin but was found to differ immunologically (Mehta and Lerman, 1971b). The subunit composition of embryonic bovine α-crystallin differs from that found in adults and subunits that are unique to the embryonic bovine lens have been described (van Kamp et al, 1974).
Examination of the water-soluble proteins of the prenatal bovine lens has shown that the relative proportions of α-, β- and γ-crystallins remain reasonably constant until birth (Francois and Rabaey, 1957; van Kamp et al., 1974).
β-Crystallin constitute a large proportion of the water soluble protein of the mammalian lens (Harding and Dilley, 1976) and can be separated into two fractions by gel filtration chromatography (Weingeist et al., 1997). These fractions are called BL (low) and BH (high).
BL is a macromolecular complex with multiple subunits of 23.5 to 30.5 kDa while BH is made of subunits of 25 to 37.5 kDa (Harding and Dilley, 1976).
Protein bands located in the range of 23-38 kDa essentially belong to different subunits of β-crystallin. In this study, the bands weighting 23.6 kDa (thus assumed to be a BL) and 34.4 kDa (which can be identified as a BH) progressively decrease with ageing, until complete disappearance in animals approximately 4-years old, in the case of last mentioned protein.
The faint band with the molecular weight of 34.4 kDa observed in this study might be related to the sheep and goat ζ-crystallin. It remains to be determined if the amino acid sequences of α-, β- and ζ-crystallins of sheep and goat are similar to those of other species studied so far. The pattern of these protein bands do not change considerably during aging of sheep and goat fetus and are not very different from those of adult animals except the 34.4 kDa protein which seems to be subjected to protelysis in adult animals.
Detailed investigations of changes in β-Crystallins during lens development and ageing have not been reported so far. However, there is some evidence that βH-crystallins are not synthesized directly, but may arise from βL-crystallins by an age-related aggregation process (Harding and Dilley, 1976).
Mammalian γ-crystallins are the smallest of crystallins. They are composed by a mixture of proteins of similar molecular weights displaying slight difference in the net charge (Harding and Dilley, 1976). As shown in SDS-PAGE pictures, many protein bands with molecular weight of 18-23 KDa are present in sheep and goat and they are very similar to the patterns reported for γ-crystallins in others species. In this study the 18.4 kDa protein, assumed to be a γ-crystallin, appeared at the 3rd month of pregnancy and gradually increased in concentration remaining constant at postnatal ages. This is compatible with the previous observation that a bovine γ-crystallin was apparently synthesized exclusively during embryonic life (Francois and Rabaey, 1957). Some bovine γ-crystallin are still synthesized in the young calf lens but very little γ-crystallin is synthesized in the adult cow (Slingsby and Croft, 1973).
The proportion of γ-crystallin remains constant throughout calf gestation, whereas the total protein concentration of the prenatal lens rises steadily, due to changes in the relative proportions of the α- and β-crystallins which rapidly increase during the first 4 months but then remain constant until birth (van Kamp et al., 1974).
The distribution of the water-soluble proteins of the prenatal human lens differs from that of the cow. In the lens of the 3 to 5 month-old human fetus α- and γ-crystallin predominate and β-crystallins, although present at this stage, appear to be synthesized in significant amounts only in the later stages of fetal life (Maisel and Goodman, 1965).
It has been generally found that with ageing the γ-crystallin proteins decreases while a proportion of the α-crystallin increases. Low levels of low-molecular weight protein, such as the γ-crystallin, were found in human lens with cataract and linked to the condition (Maisel and Goodman, 1965). Generally speaking, the neonatal human lens shows the greatest apparent complexity but the sharpness of the electrophoretic separation deteriorates with ageing (Harding and Dilley, 1976).
In most other mammals very little difference was observable between lens proteins from young and old animals (Francois and Rabaey, 1957).
Molecular weight and amino acid composition of calf (Mason and Hines, 1966), rat (Zigman, 1969) and human (Clark et al., 1969) γ-crystallins are similar. γ-crystallins make up about 1.5% of lens proteins of adult mammals, but constitute as much as 60% of the soluble proteins in weaning animals (Weingeist et al., 1971).
In conclusion the data obtained confirm that there are few notable differences between pre- and post-natal optic lens proteins in sheep and goats. As the lens proteins from different species appear to perform different functions both in- and extra-lenticularly (Piatigorsky, 2003), and behave differently with regard to their susceptibility to proteolysis by calpains and other proteases (Shearer, et al., 1997), the data obtained in this study might provide the ground for further studies to elucidate the origin of these differences.
Acknowledgment
This research was financially supported by grant 75-VE-930-547 from Shiraz University Research Council.
References
Bera, S., Ghosh, S.K., 1999. Interaction of H + ion with alpha-crytallin: saolvent accessibility of ionizable siee chains and surface charges. Biophysical Chemistry. 16, 147-160.
Bjork, I., 1961. Studies on g- crystallin. I. Isolation by gel filtration. Exp. Eye Res., 1, 145-154.
Clark, R., Zigman, S., Lerman, S., 1969. Studies on the protein of human lens. Experimental Eye Research 8, 172-182.
Denis, H.M. Brooks, D. E., Alleman, A.R., Stacy E. Andrew , S.E., Plummer, C. ,2003. Detection of anti-lens crystallin antibody in dogs with and without cataracts. Veterinary Ophthalmology 6, 321-325,
De Jong, W.W., van der Quderaa, F.J., Versteeg, M., Groenewoud, G., Van Anelsvoort, J.M., Bloemendal, H., 1975. Primary structure of the a- crystallin A chains of seven mammalian species. European Journal of Biochemistry 53, 237-242.
De Jong, W.W., Lubsen, N.H. Kraft, H.J., 1994. Molecular evolution of the eye lens. Progress in Retinal Eye Research 13, 391–442.
De Mate, H.C.; Bami, Y.P.; Korc, I.; Soluza, N.A. 1976. cellulose acetate electrophoresis of soluble lens proteins of different species of mammals: the use of discontinuous buffer systems. Comparative Biochemistry Physiology 55B, 45-48.
Evans, H.E., Sack, W.O., 1973. Prenatal development of domestic and laboratory mammals. Anatomia Histologia Embroylogia 2, 11-42.
Ferguson, W.F., Koenig, V.L. 1972. Isolation and characterization of bovine, canine and ovine α-crystallins. Comparative Biochemistry Physiology 43B, 151-161.
Francois, J., Rabaey, M., 1957. On the existence of an embryonic lens protein. Arch. Ophthalmol. 67, 672-80.
Hardings, J. Dilley, K., 1976. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp. Eye Res., 22, 1-73.
Ingolia, T.D., Craig, E.A., 1982. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proceeding of National Academy of Science, USA 79, 2360–2364.
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685.
Lien-The, K.N., Hoenders, H.J. H.M., 1974. Crystallin as an intermediate in the conversion of water-soluble into water- insoluble rabbit lens proteins. Experimental Eye Research. 19, 549-557.
Lowry, O.H., Resoenbrough, N.S., Farr, A.L., Randall, R.J., 1951. Protein measurement with folin-phenol reagent. Journal of Biological Chemistry 123, 265-275.
Maisel, H., Goodman, M., 1965. The ontogeny and specificity of human lens proteins. Invest. Ophthalmol. 4, 129-37.
Mason, C.V., Hines, M.C., 1966. Alpha-, beta- and gamma- crystallin in the ocular lens of rabbits : Preparations and partial characterization. Investigative Ophthalmology 5, 601-609.
Mehta, P.D., Lerman, S. 1971. Comparative studies on lens α-crystallin from eight species. Comparative Biochemistry Physiology 38A, 637-643.
Mehta, P.D., Lerman, S. 1971b. Comparative studies on α-crystallin during development of the bovine lens. Biochim. Biophys. Acta 236, 718-24.
Piatigorsky, J., 2003. Crystallin genes: specialization by changes in gene regulation may precede gene duplication. Structural and Functional Genomics 3 ,131–37.
Roy, B., Ghosh, S.K. 1991. Purification and properties of the low-molecular weight alpha-crystallin from normal goat lens: comparison with bovine lens. Experimental Eye Research 53, 693-701.
Shearer, T., R., Ma, H.C.,Azuma, M., 1997. Selenite nuclear cataract: Review of the model. Molecular Vision, 3, 8, http://www.emory.edu/molvis/v3/shearer.
Slingsby, C., Croft, L.R. (1973) Developmental changes in the low molecular weight proteins of the bovine lens. Exp. Eye Res. 17, 369-76.
Stauffer, J., Li, L.U., Rothschild, C., Spector, A., 1973. Fractionation and characterization of the polypeptide chains of low molecular weight calf lens alpha crystallin. Experimental Eye Research 17, 329-340.
van Kamp, G.J., Schats, L.H.M., Hoenders, H.J., 1973. Characteristics of a-crystallin related to fiber cell development in eye lenses. Biochemica. Biophysica. Acta 295, 166-173.
van Kamp, G.J., Struyker-Boudier, H.A.J., Hoenders, H.J., 1974. The soluble proteins of the prenatal bovine eye lens. Comp. Biochem. Physiol. 49B, 445-56.
van Kleef, F.S.M., Nijzink, M.J.C.M. Hoenders, H.J., 1974. Nine polypeptide chains in a-Crystallin. Experimental Eye Research 18, 201-204.
Weingeist, T.A., Liesegang, T.J., Slamovits, T.L., 1971. Biochemistry In: Basic and Clinical Science Courses. Fundamentals and Principles of Ophthalmology: Am. Acad. Ophtalmol. pp. 331-334.
Wistow, G.J. and Piatigorsky, J., 1987. Recruitment of enzymes as lens structural proteins. Science, 236, 1554–1556.
Wistow, G., Piatigorsky, J., 1988. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annual Review of Biochemistry 57, 479–504
Zigman, S., 1969. Sulphonation of rat lens. Biochemica Biophysica Acta 181, 319-322.
Figures
![]()
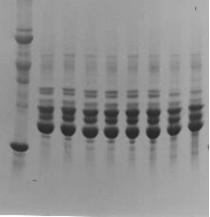
Fig. 1) DS-PAGE patterns of lens proteins of young and adult sheep. M: Molecular weight markers. 1: 3 months, M; 2: 3 months, F, 3: 6 months, M; 4: 6 months, F; 5: 1 year, M; 6: 1 year, F; 7: 4 years, M, 8: 4 years, F
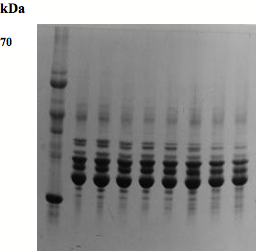
Fig. 2) SDS-PAGE pattern of lens proteins of goats on a 10-20% acrylamide gradient gel. M: Molecular weight markers. 1: 3 months, M; 2: 3 months, F; 3: 6 months, M; 4: 6 months, F; 5: one year, M; 6: one year, F; 7: 4 years, M; 8: 4 years, F.
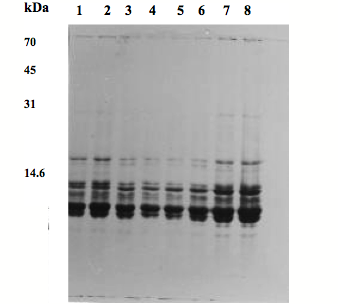
Fig. 3) SDS-PAGE patterns of fetal lens proteins of sheep on a 12.5 % acrylamide gel.
1: 2- month fetus, M; 2: 2-month fetus, F; 3: 3-month fetus, M; 4: 3-month fetus, F; 5:
4-month fetus, M; 6: 4 month fetus, F; 7: 3 –day lamb, M; 8: 3 -day lamb, F.
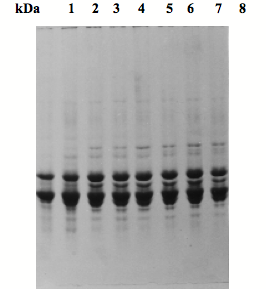
Fig. 4) SDS-PAGE patterns of lens proteins of adult sheep on a 12.5% acrylamide gel.
1: young 3-month old, M; 2: 3-month, F; 3: 6-month, M; 4: 6-month, F; 5: one year, M; 6: one year, F; 7: 4 years, M; 8: 4 years, F.
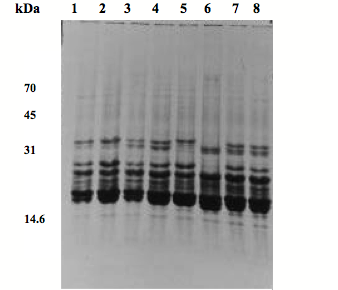
Fig. 5) SDS-PAGE patterns of fetal lens proteins of goats on a 12.5 % acrylamide gel. 1: 2-month fetus, M; 2: 2-month fetus, F; 3: 3-month fetus, M; 4: 3-month fetus, F; 5: 4-month fetus, M; 6: 4-month fetus, F; 7: 3 days goat, M; 8: 3 days goat, F
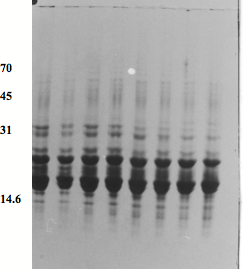
Fig. 6) SDS-PAGE patterns of lens proteins of adult goats on a 12.5% acrylamide gel. 1: 3 months, M; 2: 3 months, F; 3: 6 months, M; 4: 6 months, F; 5: one year, M; 6: one year, F; 7: 4 years, M; 8: 4 years, F.
Click
on these links to visit our Journals:
Psychiatry
On-Line
Dentistry On-Line | Vet
On-Line | Chest Medicine
On-Line
GP
On-Line | Pharmacy
On-Line | Anaesthesia
On-Line | Medicine
On-Line
Family Medical
Practice On-Line
Home • Journals • Search • Rules for Authors • Submit a Paper • Sponsor us
All pages in this site copyright ©Priory Lodge Education Ltd 1994-